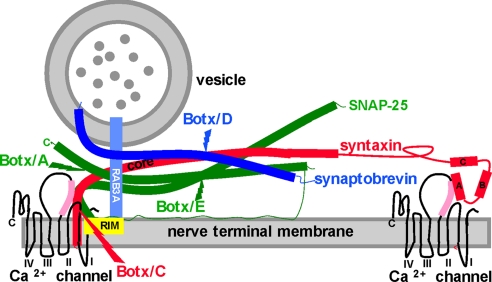
Fig. 1.
The secretory apparatus and the cleavage sites for Botxs. The SNAREs syntaxin (red), SNAP-25 (green), and synaptobrevin (darker blue) are shown in their primed state based on the crystal structure of the complex, as are the cleavage sites for Botx/A, C, D, and E (38). Some liberty has been taken with respect to the distance between vesicle and nerve terminal membrane and with the relative sizes of the HABC domains of syntaxin for illustrative purposes in this figure and in Fig. 5. For syntaxin, the helical core domain (core, also termed the H3 domain) contributes one helical domain to the parallel four-helix bundle of the primed SNAREs, with SNAP-25 (two helices) and synaptobrevin (one helix) constituting the rest of the bundle. Recent publications provide strong evidence that the Hcore domain is the likely target for GPCR activation (10, 32). The other three N-terminal helices of syntaxin (HA, B, and C) do not contribute to the SNARE complex. The reported interactions of the HA domain (32) and the Hcore (H3) domains (35) with the synprint region (pink) of P/Q- and N-type Ca2+ channels are both depicted. The synprint region and the P/Q Ca2+ channel structure were drawn in accordance with refs. 28–32. Also shown is GTP-bound Rab3A (lighter blue) interacting with RIM (yellow). Synaptotagmins (20), which are the Ca2+ sensors that mediate the action of Ca2+ once this divalent cation has entered the nerve ending via voltage gated Ca2+ channels, are not shown.