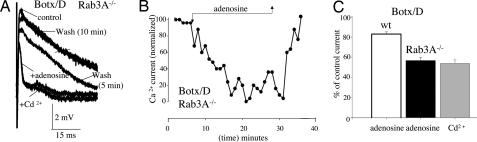
Fig. 4.
Increased efficacy of adenosine in the Rab3A−/− mutant after cleavage of the vesicle protein synaptobrevin with Botx/D. (A) Raw traces (each trace is the average of three to six stimuli, 0.017 Hz). Note the complete and reversible inhibition of Ca2+ currents by adenosine and its similarity to the effects of Cd2+. (B) Inhibition by adenosine of the normalized peak Ca2+ current. Each point is the response to a single stimulus. To normalize the peak Ca2+ current, the residual outward Cd2+-insensitive component (a component that is independent of Ca2+ entry via presynaptic Ca2+ channels; see refs. 5 and 29) was subtracted from peak currents. This residual outward current after Cd2+ is likely to be due to the Na+ current associated with the action potential, a current that passively repolarizes the part of the nerve ending under the recording electrode or an as-yet-uncharacterized ionic current (4, 5, 29). (C) Effects of adenosine after cleavage of synaptobrevin with Botx/D in Rab3A−/− mutant (filled bar) are indistinguishable from those of maximal P/Q-type calcium channel block with Cd2+ (shaded bar). In contrast, the effects of Botx/D in the wild-type mouse (wt, open bar) are indistinguishable from the effects of adenosine in the absence of toxin treatment (see Fig. 3 in ref. 19). For further details, see the text.