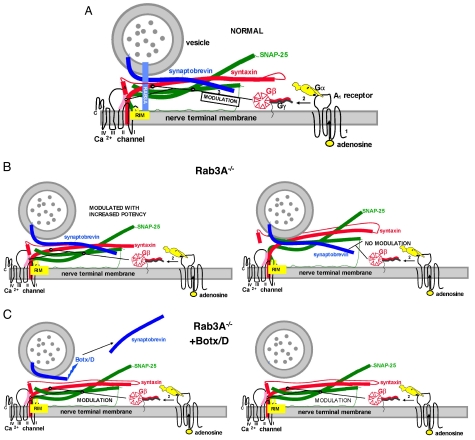
Fig. 5.
A model for the enhanced effects of adenosine before and after disruption of vesicle proteins. (A) Same conditions as Fig. 1, but with the adenosine receptor and G protein included. The depiction of the G protein includes the β propeller region of Gβ that is believed to interact with effectors and the Gγ subunit with its prenylated attachment to the membrane (39). The Gα subunit was drawn in accordance with the crystal structure, with the receptor interaction domain and the GTP-binding region shown as a black circle and a black curving line, respectively. The helical domains are shown as ear-like appendages on the canine-like subunit. This figure also shows the N terminus of RIM interacting with Rab3A and two additional domains (C2 domains) that have been shown to interact with t-SNAREs and Ca2+ channels (40). For further details of the GPCR activation scheme for adenosine, see the text. (B) The situation in the Rab3A−/− mutant. Note the change in conformation in the SNARE complex in this mutant (Left) such that the complex has a higher affinity for Gβ. The SNARE- Ca2+ channel complex (Right) is not capable of being modulated by adenosine (no modulation). (C) The Rab3A−/− mutant after cleavage of synaptobrevin with Botx/D (Left). Note the recruitment of the additional site for modulation by adenosine by changes in conformation of the t-SNARE–Ca2+ channel complex when synaptobrevin is cleaved in the mutant mouse (Right, modulation). Recruitment of this site thus increases the level of inhibition of the macroscopic calcium currents. For further details of the synaptic proteins, see Fig. 1.