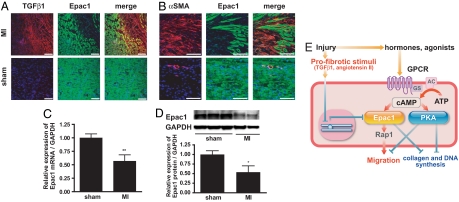
Fig. 5.
Epac1 expression is decreased after myocardial infarction in vivo. (A) Sections of cardiac intraventricular septa from rats subjected to myocardial infarction (MI) or sham operation (sham) were stained for TGFβ1 (red), Epac1 (green) and nuclei with DAPI (blue). The images of the border zone that abuts on areas of infarction are shown. (Scale bar, 100 μm.) (B) Sections of intraventricular septa of rats subjected to myocardial infarction (MI) or sham operation (sham) were stained for αSMA (red), Epac1 (green), and DNA with DAPI (blue). The images of the border zone of infarction are shown. (Scale bar, 100 μm.) (C) Cardiac fibroblasts isolated from intraventricular septa of postmyocardial infarction and sham-operated rats were cultured for 16 h to eliminate other cell types. RNA was isolated and subjected to quantitative RT-PCR. GAPDH was used as standard. n = 4–5. **, P = 0.0085 compared with the sham-operated group. (D) Expression of Epac1 protein. (Upper) Representative images of immunoblots. (Lower) Densitometry of the blots for Epac1 protein normalized to GAPDH protein expression is shown in the graph. n = 3, **, P = 0.0367. (E) Model for the role of Epac and PKA in fibroblasts after injury. After injury, levels of cAMP-elevating hormones increase, activating cognate GPCR. The enhanced synthesis of cAMP by adenylyl cyclase activation stimulates Epac and PKA. Epac promotes fibroblast migration, whereas PKA inhibits migration and fibroblast-to-myofibroblast transformation. Both Epac and PKA inhibit collagen synthesis and fibroblast proliferation. Profibrotic stimuli inhibit Epac expression and stimulate collagen synthesis.