Abstract
Ablation of germ-line precursor cells in Caenorhabditis elegans extends lifespan by activating DAF-16, a forkhead transcription factor (FOXO) repressed by insulin/insulin-like growth factor (IGF) signaling (IIS). Signals from the gonad might thus regulate whole-organism aging by modulating IIS. To date, the details of this systemic regulation of aging by the reproductive system are not understood, and it is unknown whether such effects are evolutionarily conserved. Here we report that eliminating germ cells (GCs) in Drosophila melanogaster increases lifespan and modulates insulin signaling. Long-lived germ-line-less flies show increased production of Drosophila insulin-like peptides (dilps) and hypoglycemia but simultaneously exhibit several characteristics of IIS impedance, as indicated by up-regulation of the Drosophila FOXO (dFOXO) target genes 4E-BP and l (2)efl and the insulin/IGF-binding protein IMP-L2. These results suggest that signals from the gonad regulate lifespan and modulate insulin sensitivity in the fly and that the gonadal regulation of aging is evolutionarily conserved.
Keywords: aging, endocrine regulation, reproduction, longevity, metabolism
Aside from dietary restriction, inhibition of reproduction is one of the most effective ways to extend animal lifespan (1–3). Despite the generality of this effect, the mechanisms by which reproduction regulates aging remain unknown (4–6). Progress toward this goal has been made with the nematode Caenorhabditis elegans (7–9). Ablation of germ-line precursor cells is sufficient to extend lifespan, and overproliferation of germ cells (GCs) shortens lifespan. In contrast, ablation of the entire gonad has no impact on longevity (7–8). These observations suggest that there is a balance between longevity assurance signals from the somatic gonad and signals from the germ line that promote aging (7). Longevity extension by germ-line ablation depends on DAF-16, the C. elegans ortholog of the forkhead transcription factor (FOXO), which is also required for longevity extension by reduced insulin/insulin-like growth factor (IGF) signaling (IIS) (7–8). To date, however, little is known about how signals from reproductive tissues systemically affect lifespan and whether the model developed in C. elegans is relevant to animals beyond the nematode (4–6, 9). Here we investigate this problem in the fruit fly, Drosophila melanogaster.
Several methods to inhibit reproduction extend Drosophila lifespan: removing oviposition substrate (10), reducing egg production (10–13), and inhibiting mating (14, 15). However, reproduction can also be reduced without affecting lifespan (3, 16–19), and whether loss of GCs extends fly lifespan remains unclear (4). Irradiation or the female-sterile mutation ovoD1 induce sterility and extend lifespan (12, 13), but whether these manipulations do so because they damage GCs or disrupt processes upstream of germ-line activity is unknown (4, 20, 21). Interestingly, a recent study suggests that GC ablation might not extend lifespan in D. melanogaster (21). Failure to form primordial GCs in grandchildless-like mutants (tudor, germ cell-less, oskar) increases lifespan only slightly or not at all (ref. 21 and our unpublished data). However, this finding is at odds with the observation that lack of a primordial germ line in a Drosophila subobscura grandchildless mutant extends lifespan (11, 22). Thus, in contrast to the worm, how reproductive processes modulate aging in the fly remains poorly understood (3–5, 23).
One reason for the discrepancies in fly studies might be that some grandchildless-like mutations impact the development of the somatic gonad (21, 24), perhaps precluding the capacity of this tissue to produce longevity assurance signals (7, 21). If so, GCs might modulate aging, but only when the somatic gonad has matured in the presence of the germ line during development. Moreover, because grandchildless-like mutants act during development (25), their impact on adult demography might involve pleiotropic effects independent of aging (5, 21). We therefore sought an alternative system that eliminates GCs exclusively in late development or the adult to test whether the D. melanogaster germ line modulates aging.
Here we investigate the impact of GC loss induced through misexpression of bag of marbles (bam). In females, bam is necessary and sufficient for differentiation of GCs, and overexpression of bam+ in GCs leads to precocious differentiation and subsequent loss of GCs (26–28). In males, bam limits mitotic amplification divisions of spermatogonia, which occur before the initiation of terminal differentiation into spermatocytes. Overexpression of bam+ in early male GCs causes GC loss, presumably through apoptosis (29). By manipulating bam, we investigate the impact of GC ablation on aging and find that loss of GCs in female and male flies extends lifespan and modulates insulin signaling.
Results and Discussion
Ectopic misexpression of bam+ in the female germ line, by using the binary GAL4>UAS system or heat shock-induction, eliminates GCs (Fig. 1) (27, 28). Previous data suggest that the lost GCs are germ-line stem cells (GSCs): heat shock-induced bam+ expression causes GC loss, but GCs that were not GSCs at the time of heat shock develop normally (27). Although grandchildless-like mutants lack pole cells and cannot form primordial GCs (21–22, 25, 30), heat shock-induced bam+ overexpression eliminates female GSCs in the third larval instar (L3) or later but not before the L3 stage (27). When driving constitutive overexpression of UASp-bam+ (28) with the germ-line-specific nanos (nos)-GAL4::VP16 driver (31), we found that GC loss continues in adult females, after the ovary has completed development. Females initially have the capacity to lay a small number of eggs but become fully sterile by day 7 [Fig. 1 and supporting information (SI) Fig. S1]. Similarly, in males, bam+ overexpression induced GC depopulation in the L3 stage or later (Fig. 1) (29). Moreover, bam+ overexpression caused a dramatic expansion of somatic cells in ovaries and testes (Fig. 1), reminiscent of the enlarged somatic gonads of agametic grandchildless-like mutants (21, 24). Thus, grandchildless-like mutants and flies misexpressing bam+ have expanded somatic gonads but complete GC loss at different times.
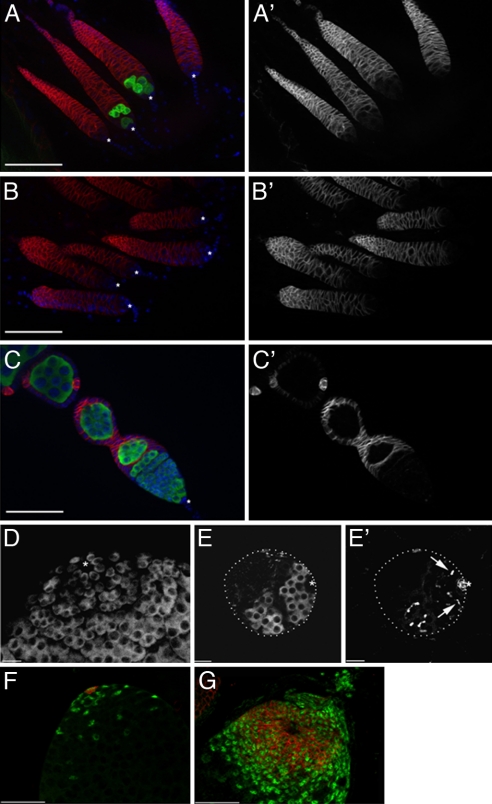
Fig. 1.
GC loss and expansion of somatic cells in gonads from flies misexpressing bam+. (A–C) GC loss in adult females misexpressing bam+ in the germ line (y w/w1118;UASp-bam+::gfp/+; nos-GAL4::VP16/+). GCs are stained for GC specific antigen vasa (green), somatic cells (FasIII, red), and DNA (DAPI, blue). Asterisk (*) denotes somatic cap cells. (A) GCs are rarely observed in ovarioles from 2-day-old females overexpressing bam+ [24/225 ovarioles (10.7%) contained GCs]. (B) GC loss is virtually complete by 7 days [3/325 ovarioles (0.9%) contained GCs]. See also Fig. S1. (C) Germarium from a 2-day-old control female. All ovarioles from control females contained GCs at 2 (n = 120) and at 7 days (n = 152). (A′–C′) shows FasIII+ somatic cells only. Note the expanded somatic gonad in GC-less females. (D and E) GC loss in third instar larval (L3) males overexpressing bam+. Control testes from L3 males (D) have a normal number of GSCs (vasa) in contact with hub cells (*) at the apical tip. (E) Males overexpressing bam+ show loss of GSCs by this stage. (E′) GCs present near the hub have branched fusomes (arrows) as detected by staining with antibodies to α-spectrin. Note the reduced size of the gonad. Asterisk (*) denotes the FasIII+ apical hub. (F and G) Expansion of somatic cells in testes from adults misexpressing bam+. (F) Control testes show normal distribution of FasIII+ hub cells (red) and TJ+ somatic cells (green). (G) An expansion of FasIII+ and TJ+ somatic cells is observed in testes. (Scale bars: 50 μm in A–C, F, and G; 20 μm in D and E.)
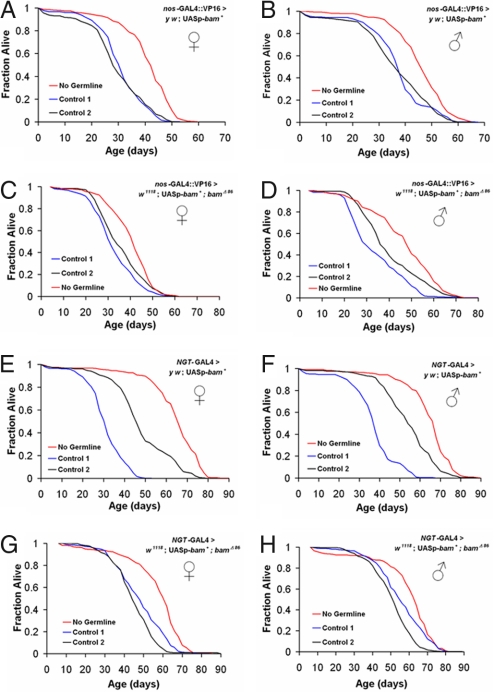
GC loss induced by misexpression of bam+ significantly increased lifespan in females and males, in several independent experiments (Fig. 2 and Table S1). Lifespan was increased by 31.3% and 50% in females and 21% and 27.8% in males by GC ablation in a y w background by driving y w;UASp-bam+ with nos-GAL4::VP16; effects are relative to a coisogenic control (y w;UASp-bam+; control 1) and a control with a heterozygous background (y w/w1118; nos-GAL4::VP16; control 2) (Fig. 2 A and B and Table S1). Longevity was also extended when UASp-bam+ was driven by nos-GAL4::VP16 in an independent background (w1118) lacking one copy of genomic bam (Fig. 2 C and D; Table S1). The capacity for GC ablation to extend lifespan was likewise effective with the germ-line driver nos-GAL4-tubulin (NGT-GAL4) (32) in the y w and w1118 backgrounds (Fig. 2 E–H and Table S1). Thus, bam+ misexpression in the germ line is sufficient to force GC loss and to increase lifespan in multiple genetic backgrounds and with different germ-line drivers. Because the failure of grandchildless-like mutants to develop GCs has no consistent major effects on lifespan (ref. 21 and our unpublished data), we hypothesize that GC loss during late development or in the adult might promote longevity because GCs associate and interact with somatic cells before loss.
Fig. 2.
Adult GC loss extends lifespan in D. melanogaster. (A–D) Driving UASp-bam+ in germ line (no germ line, UASp-bam+/+; nos-GAL4::VP16/+) extends lifespan, both in a y w background (A, females; B, males) and a w1118 background lacking one copy of bam (C, females; D, males), relative to two controls. Controls were, in the y w background, y w/y w;UASp-bam+::gfp/+ (control 1) and y w/w1118; nos-GAL4::VP16/+ (control 2), and in the w1118 background, w1118/w1118;UASp-bam+::gfp/+; bamΔ86/+ (control 1) and w1118/w1118; nos-GAL4::VP16/+ (control 2). (E–H) Misexpressing UASp-bam+ with an alternative germ-line-specific driver, nos-GAL4-tubulin (UASp-bam+::gfp/NGT-GAL4), also extends lifespan, both in the y w background (E, females; F, males) and in a background lacking one copy of bam (G, females; H, males) compared with two controls (control 1: y w/y w;UASp-bam+::gfp/+ or w1118/y w;UASp-bam+::gfp/+; bamΔ86/+, respectively; control 2: y w/y1 w*; NGT-GAL4/+). See Table S1 for statistics.
If the germ line produces a signal that shortens lifespan or represses a somatic signal that extends lifespan, GC overproliferation should decrease lifespan (7). To test this prediction, we examined a sterile heteroallelic null mutant of bam (bamΔ86/bamΔ59) in which mitotically active, nondifferentiating GSCs overproliferate (26). Mutant flies were short-lived relative to two fertile controls (Fig. S2 and Table S1). Thus, eliminating GC proliferation slows aging, whereas GC overproliferation shortens lifespan in the fly, as in the nematode (7, 8). However, we cannot fully exclude the possibility that the longevity effects of bam are independent of its effects on GCs.
Germ-line loss might slow aging simply by abolishing the survival costs of producing gametes (1–2, 4, 21, 23). To rule out that egg production is required for GCs to shorten lifespan, we examined a female-sterile mutant of egalitarian (egl) (33). Mutants of egl prevent differentiation of cystoblasts into oocytes (34). Consequently, flies produce eggs with 16 rather than 15 nurse cells, and egg chambers degenerate before they acquire yolk (34). Lifespan of sterile egl mutant females (eglPR29/eglwu50) was reduced compared with fertile controls (Fig. S3 and Table S1), suggesting that oogenesis per se might not be sufficient for reproduction to shorten lifespan. This result adds to a growing number of cases showing that the tradeoff between reproduction and survival can be decoupled (3, 16–19, 21, 23).
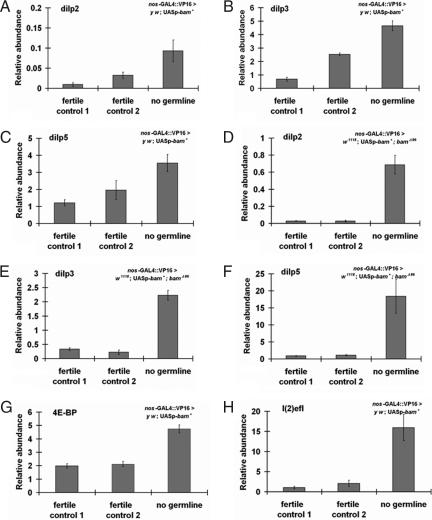
In C. elegans, lifespan extension by GC loss requires the FOXO transcription factor DAF-16; FOXO activity is normally repressed by IIS (7–8). Because reduced IIS slows Drosophila aging [by mutations disrupting IIS (13, 35), constitutive activation of Drosophila FOXO (dFOXO) (18), or ablation of insulin-producing cells (36, 37)], we reasoned that GC loss might extend lifespan by down-regulating IIS. Accordingly, we measured message abundance for the three Drosophila insulin-like peptides (dilps) produced by median neurosecretory cells (mNSCs), the major insulin-producing cells (IPCs) in the brain of the adult (Fig. S4A) (38–40). Rather than reduced message from the dilp2, dilp3, and dilp5 loci, we found that these transcripts were induced upon GC loss by 1.8- to 26-fold relative to controls, in two independent genetic backgrounds (Fig. 3 A–C and D–F).
Fig. 3.
GC loss up-regulates dilp message but activates expression of dFOXO target genes. (A–D) GC-less flies (UASp-bam+/+; nos-GAL4::VP16/+) exhibit increased production of dilp 2, dilp 3, and dilp 5, both in the y w (A–C) and w1118 backgrounds (D–F), relative to controls. (G–H) GC loss causes up-regulation of dFOXO targets 4E-BP (G) and l (2)efl (H) in both backgrounds. For details of genotypes, see Fig. 1.
Previous attempts to quantify DILPs by Western blot analysis have failed because of low ligand abundance (37), and current technology does not permit detection of circulating DILPs in the hemolymph. However, several observations suggest that increased dilp message in GC-ablated flies might be biologically meaningful. Immunostaining of brains with DILP antibody indicated that the IPCs of GC-less flies produced as much and, in some cases, more DILP protein than controls, and DILP+ staining of IPC axonal projections was strong, suggesting functional DILP transport (Fig. S4 B–E). Furthermore, neural DILPs homeostatically regulate sugar levels in the hemolymph (37, 40), and GC-less flies had reduced amounts of stored and circulating carbohydrates (Fig. S5 A and B).
The hyperinsulinism of GC-less flies is a paradox because lifespan should not be extended in the face of increased DILPs. Because high DILP levels should activate IIS in peripheral tissues and repress dFOXO, we measured transcripts of two major dFOXO targets from body tissue, the translational regulator thor (encoding 4E-BP), and the small heat shock protein l (2)efl, which are normally induced when IIS is repressed and dFOXO is activated (41–43). Message levels of both dFOXO targets were up-regulated in GC knockout flies (Fig. 3 G and H). Although we cannot rule out that these targets have transcriptional inputs other than dFOXO (44), flies with GC loss, despite elevated DILPs, express markers consistent with active dFOXO and reduced IIS.
Because reduced IIS causes dephosphorylation and nuclear translocation of dFOXO, nuclear accumulation of dFOXO can be used to assess IIS pathway activity. To confirm that dFOXO is active in GC-less flies, we examined its localization with immunostaining in peripheral fat body, a major site of IIS activity, and by Western blotting analysis with cell fractionation in whole-body tissue (Figs. S6 and S7). As expected, dFOXO was predominantly nuclear in GC flies, indicating that dFOXO is active. Yet, despite differential up-regulation of dFOXO targets, GC-less and control flies did not differ in nuclear dFOXO localization (Figs. S6 and S7), which suggests that GC loss might affect dFOXO activity independent of its subcellular localization, as recently found in C. elegans (45).
There are many mechanisms by which IIS can be impeded between the site of insulin production and FOXO-dependent responses of peripheral tissues: at the level of insulin secretion or transport and at many steps within intracellular IIS of target tissues (46, 47). To initiate an understanding of IIS impedance in GC-less flies, we explored whether GC loss might change transcript abundance of two DILP cofactors, dALS and IMP-L2 (48–50). In mammals, circulating IGFs form a complex consisting of IGF-1, IGF-binding proteins (IGF-BPs), and the liver-secreted scaffold protein acid labile substrate (ALS); by creating a pool of circulating IGFs, this ternary complex limits ligand availability (51). The Drosophila homolog of ALS (dALS) is expressed in DILP-expressing IPCs and the fat body (48, 52) and is up-regulated in dFoxo null mutants (53). Consistent with the model that dALS functions as a DILP cofactor, dALS forms a circulating trimeric complex containing DILP2 and IMP-L2, an Ig-like homolog of IGF-BP7 (48, 54). Binding of dALS requires prior formation of a dimeric complex containing DILP2 and IMP-L2 (48). In cell culture experiments, IMP-L2 binds mammalian insulin and IGF-1/-2, and fall army worm (Spodoptera frugiperda) IMP-L2 inhibits IIS through the human insulin receptor (55). Because overexpression of dALS and IMP-L2 can systemically antagonize DILP function and IIS in Drosophila in vivo (48, 49), we measured message abundance of dALS and IMP-L2 upon GC loss. Although dALS levels did not change, IMP-L2 message was increased 7-fold in GC-less flies (Fig. 4 A and B). Although this observation is correlational, it might suggest a potential explanation for why IIS might be impeded in GC-less flies in the face of elevated DILP production. It will be of major interest to determine whether GC loss can modulate DILP availability and IIS by affecting IMP-L2.
Fig. 4.
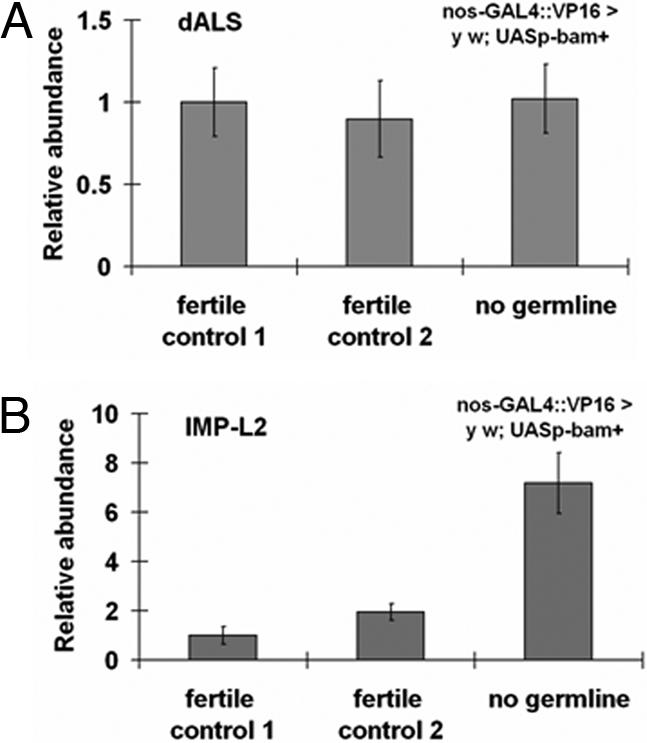
GC loss up-regulates message of IMP-L2, but not of dALS. (A) GC loss does not alter message abundance of the DILP cofactor dALS. (B) In contrast, GC loss strongly up-regulates expression of the insulin/IGF- binding protein IMP-L2. See Fig. 1 for genotype information.
Together, our results show that GCs regulate aging and modulate IIS in the fly. Although future work is required to fully characterize IIS state upon GC loss, we observed that GC-less flies exhibit characteristics of both increased and decreased IIS. Increased DILPs and hypoglycemia are suggestive of increased IIS, but GC-less flies also have markers of IIS impedance. The induction of dFOXO targets is consistent with the finding that lifespan extension by GC loss in the nematode requires FOXO/DAF-16 (7–8). In the worm, GC loss induces nuclear translocation of DAF-16 and activates DAF-16 targets, but nuclear accumulation is also observed in worms that lack the entire gonad and have normal lifespan (45). Similarly, we find that GC-less and control flies differ in dFOXO target activation, but not dFOXO localization, suggesting that IIS can affect aging by modulating FOXO/DAF-16 activity independent of subcellular localization. Indeed, dietary restriction in C. elegans extends longevity by activating AMP-activated protein kinase (AMPK), which phosphorylates and activates DAF-16 but does not promote DAF-16 nuclear translocation (56).
Because extended longevity by GC loss is associated with up-regulation of DILPs, GC loss might impede IIS downstream of DILP production. In humans, compensatory hyperinsulinemia is a hallmark of severe insulin resistance (57), and mutations in the tyrosine kinase domain of the insulin receptor can cause hyperinsulinemic hypoglycemia coupled with insulin resistance (58). Recent studies with fly and mouse also suggest that lifespan can be extended despite hyperinsulinemia (59, 60). In Drosophila target-of-rapamycin (dTOR) mutants, longevity extension is associated with elevated DILP2 and hypoglycemia (59), and brain-specific insulin receptor substrate-2 (Irs-2) knockout mice are hyperinsulinemic but insulin-resistant and long-lived (60). Clearly, further experiments are needed to unravel the mechanisms by which insulin production can be uncoupled from IIS sensitivity and modulation of lifespan.
Our finding that GC loss affects neural DILP production also adds to growing evidence suggesting evolutionary conservation of endocrine feedback between brain and gonad (61). In Drosophila, neural DILPs bind to the insulin-like receptor (dINR) on GSCs to regulate GC proliferation (62, 63), and neuronal InR knockout (NIRKO) mice show impaired spermatogenesis and ovarian follicle maturation (64). Conversely, in rats, ovariectomy decreases IGF-1 receptor density in the brain but increases circulating IGF-1 levels (65). Together with progress made in the worm (7,8–9, 66) and mouse (67), the Drosophila system will allow us to dissect the mechanisms underlying the fundamental and intricate relationship among IIS, reproduction, and aging.
Materials and Methods
Fly Strains and Maintenance.
For bam+ misexpression, we used UASp-bam+ in a y w background, obtained by backcrossing w; [w+;UASp-bam+::gfp]/CyO; bamΔ86/TM3 (28) for six generations into y w and eliminating the bam mutant allele. We also misexpressed bam+ in a w1118 background lacking one copy of bam (w; [w+;UASp-bam+::gfp]/CyO; bamΔ86/TM3). For each UAS responder, we induced expression with two germ-line-specific nanos (nos)-GAL4 lines (w1118; +/+; nos-GAL4::VP16 [MVD1] and y1 w*; NGT-GAL4 [nos-GAL4-tubulin]) (31, 32). Thus, we examined how bam+ misexpression affects lifespan in four sets of genotypes: (i) no germ line: y w/w1118;UASp-bam+::gfp/+; nos-GAL4::VP16/+; control 1: y w/y w;UASp-bam+::gfp/+; control 2: y w/w1118; nos-GAL4::VP16/+; (ii) no germ line: w1118/w1118;UASp-bam+::gfp/+; nos-GAL4::VP16/bamΔ86; control 1: w1118/w1118;UASp-bam+::gfp/+; bamΔ86/+; control 2: w1118/w1118; nos-GAL4::VP16/+ (control 2); (iii) no germ line: y w/y1 w*;UASp-bam+::gfp/NGT-GAL4; control 1: y w/y w;UASp-bam+::gfp/+; control 2: y w/y1 w*; NGT-GAL4 (Fig. 2 E and F); and (iv) no germ line: w1118/y1 w*;UASp-bam+::gfp/NGT-GAL4; bamΔ86/+; control 1: w1118/y w;UASp-bam+::gfp/+; bamΔ86/+; control 2: y w/y1 w*; NGT-GAL4/+. For the assay in Fig. S2, we used a heteroallelic null mutant (ry506 e1 bamΔ86/red e bamΔ59) and two heterozygous controls (w1118/+; red e bamΔ59/+ and w1118/+; ry506 e1 bamΔ86). bamΔ59 is an undescribed deletion, and bamΔ86 is described in ref. 26. The assay in Fig. S3 was performed with a heteroallelic mutant (w; cn bw eglPR29/cn bw eglwu50) and a control overexpressing egl+ in the mutant (w; cn bw eglPR29/cn bw eglwu50; CA8B(egl+)/+) (33, 34). UASp-bam+, bam mutants, and w1118 were donated by D. McKearin (University of Texas Southwestern Medical Center, Dallas); y w by E. Rulifson (University of California, San Francisco); nos-GAL4::VP16 and egl by R.L.; NGT-GAL4 strain by the Bloomington Stock Center (Bloomington, IN); dilp2-GAL4 by E. Hafen (ETH Zürich); and UAS-CD8::gfp by R. Stocker (Université de Fribourg, Fribourg, Switzerland). Flies were reared and experiments were conducted at 25°C and 40% relative humidity on a 12-hour light–dark cycle and using a standard cornmeal/sugar/yeast/agar diet.
Gonad Immunocytochemistry.
Immunofluorescence experiments on squashed testes were performed as described in ref. 29. Ovaries were dissected into PBS, fixed in fresh 4% formaldehyde/PBS for 30 min, and blocked in PAT (PBS/0.1% Triton X-100/1% BSA) for 2 h at room temperature. Primary and secondary antibodies were diluted in PAT; incubation with primary antibodies was carried out overnight at 4°C. Ovaries were washed quickly twice, followed by four 30-min washes at room temperature in PBT (PBS with 0.1% Triton X-100). Primary antibodies used were mouse monoclonal anti-fasciclin III (FasIII) (7G10) and anti-α-spectrin (3A9) at 1:10 (Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA), rabbit anti-Vasa at 1:2,000 (gift from P. Lasko, McGill University, Montreal, QC), and guinea pig anti-Traffic jam (Tj) at 1:3,000 (gift from D. Godt, University of Toronto, Toronto, ON). Secondary antibodies were obtained from Molecular Probes. Samples were mounted in Vectashield medium with DAPI (Vector Laboratories). Images were obtained by using a Zeiss Axiovert 200 microscope and processed with Zeiss AxioVision (version 4.5) and Adobe Photoshop software.
Lifespan Assays.
Adult survival was determined by using previously described methods (23, 35, 68). Newly eclosed adult flies were collected within a 24-hour period. To minimize stress-induced mortality in very young flies, we lightly anesthetized flies with moist CO2. Each 1-liter demography cage (68) was initiated with ≈150 newly eclosed adults, mixed sexes (unless otherwise noted; Table S1). Dead flies were recorded and removed every 2 days, at which time fresh food was provided in a vial with 3 ml of medium. We used four to five replicate cages for each treatment/genotype; data were combined across replicates for each treatment/genotype. Survival, lx, was estimated as Nx/N0, where Nx is the number of flies alive at the beginning of each census interval and N0 is the initial cohort size (69). We tested for significant differences in survival between pairs of cohorts using log-rank tests (69). Data were analyzed with JMP (SAS Institute) (70). We also inspected and analyzed patterns of age-specific mortality (69) to verify that differences in survival were caused by continuous differences in mortality rate (data not shown).
Quantitative PCR (qPCR).
mRNA transcript levels were measured with reverse-transcription qPCR. Ten-day-old live females were snap-frozen in liquid nitrogen and stored at −80°C. Heads were separated from bodies by using a funnel with a fine mesh. For neural dilps, we measured message from heads, whereas for all other transcripts we measured message from decapitated bodies. Because heads can thaw rapidly and mRNA degrades, all sample preparations were performed with iced reagents and containers before RNase inactivation. We prepared total RNA from three to four replicates per genotype, each replicate with 75 heads or bodies, using TRIzol reagent (Invitrogen). The purity and amount of RNA was determined spectrophotometrically (NanoDrop, ND-1000). DNase-treated total RNA was reverse-transcribed by using the iScript cDNA synthesis kit (Bio-Rad) according to the manufacturer's protocol. For qPCR we used iTaq SYBR Green Supermix with ROX (Bio-Rad) and an ABI prism 7300 Sequence Detection System (Applied Biosystems). Each PCR was performed by using three to four biological replicates; each biological replicate was replicated three times (technical replicates). For each transcript, we normalized message levels relative to a GAPDH2 control by the method of 2−ΔΔCT (71). Previous work, confirmed by independent microarray analysis, suggested that GAPDH2 is a robust control when analyzing dilp transcript levels (18); statistical analysis of CT values of GAPDH2 controls confirmed that GAPDH2 did not differ among genotypes (Fig. S8). For information on primers see SI Materials and Methods.
Fecundity Assay.
Details of the fecundity assay are described in SI Materials and Methods.
Carbohydrate Measurements.
Hemolymph and total carbohydrates were measured as described (37, 40). For details see SI Materials and Methods.
DILP Immunocytochemistry.
DILPs were detected by immunostaining of brains with DILP antibody as described (19, 72). Details are given in SI Materials and Methods.
FOXO Immunocytochemistry and Western Blot Analysis.
We examined dFOXO subcellular localization with anti-dFOXO antibody in peripheral fat body tissue, as described in ref. 18, and in nuclear and cytosolic extracts of whole-body samples by Western blotting. Details are given in SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank H. Broihier, M. Brown, D. Godt, E. Hafen, P. Lasko, D. McKearin, E. Rulifson, R. Stocker, the Bloomington Stock Center, and the Developmental Studies Hybridoma Bank at the University of Iowa for reagents and fly stocks; J. Bauer, R. Butler, S. Morris, S. Megumi-Naylor, M. Rocha, B. Sage, D. Warburton, and R. Yamamoto for technical assistance; P. Léopold, H. Stocker, and E. Hafen for sharing results before publication; and two referees for helpful comments on the manuscript. This work was supported by National Institute on Aging (National Institutes of Health) Grants AG024360 and AG021953 (to M.T.). R.L. is a Howard Hughes Medical Institute Investigator; D.L.J. is an Ellison Medical Foundation (EMF) New Scholar; and M.T. is an EMF Senior Scholar. This research was conducted while T.F. was a Roche Research Foundation Fellow and a Swiss National Science Foundation Postdoctoral Fellow.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0709128105/DCSupplemental.
References
- 1.Bell G, Koufopanou V. In: Oxford Surveys in Evolutionary Biology. Dawkins R, Ridley M, editors. Vol 3. Oxford: Oxford Univ Press; 1986. pp. 83–131. [Google Scholar]
- 2.Reznick D. Costs of reproduction: An evaluation of the empirical evidence. Oikos. 1985;44:257–267. [Google Scholar]
- 3.Partridge L, Gems D, Withers DJ. Sex and death: What is the connection? Cell. 2005;120:461–472. doi: 10.1016/j.cell.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 4.Leroi A. Molecular signals versus the loi de balancement. Trends Ecol Evol. 2001;16:24–29. doi: 10.1016/s0169-5347(00)02032-2. [DOI] [PubMed] [Google Scholar]
- 5.Harshman LG, Zera AJ. The cost of reproduction: The devil in the details. Trends Ecol Evol. 2007;22:80–86. doi: 10.1016/j.tree.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 6.Tatar M. Germ-line stem cells call the shots. Trends Ecol Evol. 2002;17:297–298. [Google Scholar]
- 7.Hsin H, Kenyon C. Signals from the reproductive system regulate the lifespan of C. elegans. Nature. 1999;399:362–366. doi: 10.1038/20694. [DOI] [PubMed] [Google Scholar]
- 8.Arantes-Oliveira N, Apfeld J, Dillin A, Kenyon C. Regulation of life-span by germ-line stem cells in Caenorhabditis elegans. Science. 2002;295:502–505. doi: 10.1126/science.1065768. [DOI] [PubMed] [Google Scholar]
- 9.Mukhopadhyay A, Tissenbaum HA. Reproduction and longevity: Secrets revealed by C. elegans. Trends Cell Biol. 2007;17:65–71. doi: 10.1016/j.tcb.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Partridge L, Green A, Fowler K. Effects of egg-production and of exposure to males on female survival in Drosophila melanogaster. J Insect Physiol. 1987;33:745–749. [Google Scholar]
- 11.Maynard Smith J. The effects of temperature and of egg-laying on the longevity of Drosophila subobscura. J Exp Biol. 1958;35:832–842. [Google Scholar]
- 12.Sgro CM, Partridge L. A delayed wave of death from reproduction in Drosophila. Science. 1999;286:2521–2524. doi: 10.1126/science.286.5449.2521. [DOI] [PubMed] [Google Scholar]
- 13.Clancy DJ, et al. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 2001;292:104–106. doi: 10.1126/science.1057991. [DOI] [PubMed] [Google Scholar]
- 14.Fowler K, Partridge L. A cost of mating in female fruitflies. Nature. 1989;338:760–761. [Google Scholar]
- 15.Chapman T, Liddle LF, Kalb JM, Wolfner MF, Partridge L. Cost of mating in Drosophila melanogaster females is mediated by male accessory gland products. Nature. 1995;373:241–244. doi: 10.1038/373241a0. [DOI] [PubMed] [Google Scholar]
- 16.Marden JH, Rogina B, Montooth KL, Helfand SL. Conditional tradeoffs between aging and organismal performance of Indy long-lived mutant flies. Proc Natl Acad Sci USA. 2003;100:3369–3373. doi: 10.1073/pnas.0634985100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simon AF, Shih C, Mack A, Benzer S. Steroid control of longevity in Drosophila melanogaster. Science. 2003;299:1407–1410. doi: 10.1126/science.1080539. [DOI] [PubMed] [Google Scholar]
- 18.Hwangbo DS, Gersham B, Tu M-P, Palmer M, Tatar M. Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature. 2004;429:562–566. doi: 10.1038/nature02549. [DOI] [PubMed] [Google Scholar]
- 19.Tu M-P, Tatar M. Juvenile diet restriction and the aging and reproduction of adult Drosophila melanogaster. Aging Cell. 2003;2:327–333. doi: 10.1046/j.1474-9728.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- 20.Extavour C, Garcia-Bellido A. Germ cell selection in genetic mosaics in Drosophila melanogaster. Proc Natl Acad Sci USA. 2001;98:11341–11346. doi: 10.1073/pnas.201409198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barnes AI, Boone JM, Jacobson J, Partridge L, Chapman T. No extension of lifespan by ablation of germ line in Drosophila. Proc R Soc London Ser B. 2006;273:939–947. doi: 10.1098/rspb.2005.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spurway H. Genetics and cytology of Drosophila subobscura IV: An extreme example of delay in gene action, causing sterility. J Genet. 1948;49:126–140. doi: 10.1007/BF02986830. [DOI] [PubMed] [Google Scholar]
- 23.Flatt T, Kawecki TJ. Juvenile hormone as a regulator of the trade-off between reproduction and life span in Drosophila melanogaster. Evolution (Lawrence, Kans) 2007;61:1980–1991. doi: 10.1111/j.1558-5646.2007.00151.x. [DOI] [PubMed] [Google Scholar]
- 24.Margolis J, Spradling A. Identification and behavior of epithelial stem cells in the Drosophila ovary. Development. 1995;121:3797–3807. doi: 10.1242/dev.121.11.3797. [DOI] [PubMed] [Google Scholar]
- 25.Boswell RE, Mahowald AP. Tudor, a gene required for assembly of the germ plasm in Drosophila melanogaster. Cell. 1985;43:97–104. doi: 10.1016/0092-8674(85)90015-7. [DOI] [PubMed] [Google Scholar]
- 26.McKearin DM, Ohlstein B. A role for the Drosophila bag-of-marbles protein in the differentiation of cystoblasts from germline stem cells. Development. 1995;121:2937–2947. doi: 10.1242/dev.121.9.2937. [DOI] [PubMed] [Google Scholar]
- 27.Ohlstein B, McKearin DM. Ectopic expression of the Drosophila bam protein eliminates oogenic germline stem cells. Development. 1997;124:3651–3662. doi: 10.1242/dev.124.18.3651. [DOI] [PubMed] [Google Scholar]
- 28.Chen D, McKearin DM. A discrete transcriptional silencer in the bam gene determines asymmetric division of the Drosophila germline stem cell. Development. 2003;130:1159–1170. doi: 10.1242/dev.00325. [DOI] [PubMed] [Google Scholar]
- 29.Schulz C, et al. A misexpression screen reveals effects of bag-of-marbles and TGFβ class signaling on the Drosophila male germ-line stem cell lineage. Genetics. 2004;167:707–723. doi: 10.1534/genetics.103.023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williamson A, Lehmann R. Germ cell development in Drosophila. Annu Rev Cell Dev Biol. 1996;12:365–391. doi: 10.1146/annurev.cellbio.12.1.365. [DOI] [PubMed] [Google Scholar]
- 31.Van Doren M, Williamson AL, Lehmann R. Regulation of zygotic gene expression in Drosophila primordial germ cells. Curr Biol. 1998;8:243–246. doi: 10.1016/s0960-9822(98)70091-0. [DOI] [PubMed] [Google Scholar]
- 32.Tracey WD, Jr, Ning X, Klingler M, Kramer SG, Gergen JP. Quantitative analysis of gene function in the Drosophila embryo. Genetics. 2000;154:273–284. doi: 10.1093/genetics/154.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schupbach T, Wieschaus E. Female sterile mutations on the 2nd chromosome of Drosophila melanogaster. 2 Mutations blocking oogenesis or altering egg morphology. Genetics. 1991;129:1119–1136. doi: 10.1093/genetics/129.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Navarro C, Puthalakath H, Adams JM, Strasser A, Lehmann R. Egalitarian binds dynein light chain to establish oocyte polarity and maintain oocyte fate. Nat Cell Biol. 2004;6:427–435. doi: 10.1038/ncb1122. [DOI] [PubMed] [Google Scholar]
- 35.Tatar M, et al. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science. 2001;292:107–110. doi: 10.1126/science.1057987. [DOI] [PubMed] [Google Scholar]
- 36.Wessells RJ, Fitzgerald E, Cypser JR, Tatar M, Bodmer R. Insulin regulation of heart function in aging fruit flies. Nat Genet. 2004;36:1275–1281. doi: 10.1038/ng1476. [DOI] [PubMed] [Google Scholar]
- 37.Broughton SJ, et al. Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc Natl Acad Sci USA. 2005;102:3105–3110. doi: 10.1073/pnas.0405775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brogiolo W, et al. An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr Biol. 2001;11:213–221. doi: 10.1016/s0960-9822(01)00068-9. [DOI] [PubMed] [Google Scholar]
- 39.Ikeya T, Galic M, Belawat P, Nairz K, Hafen E. Nutrient-dependent expression of insulin-like peptides from neuroendocrine cells in the CNS contributes to growth regulation in Drosophila. Curr Biol. 2002;12:1293–1300. doi: 10.1016/s0960-9822(02)01043-6. [DOI] [PubMed] [Google Scholar]
- 40.Rulifson EJ, Kim SK, Nusse R. Ablation of insulin-producing neurons in flies: Growth and diabetic phenotypes. Science. 2002;296:1118–1120. doi: 10.1126/science.1070058. [DOI] [PubMed] [Google Scholar]
- 41.Puig O, Marr MT, Ruhf ML, Tjian R. Control of cell number by Drosophila FOXO: Downstream and feedback regulation of the insulin receptor pathway. Genes Dev. 2003;17:2006–2020. doi: 10.1101/gad.1098703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teleman AA, Hietakangas V, Sayadian AC, Cohen SM. Nutritional control of protein biosynthetic capacity by insulin via Myc in Drosophila. Cell Metab. 2008;7:21–31. doi: 10.1016/j.cmet.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 43.Wang MC, Bohmann D, Jasper H. JNK extends life span and limits growth by antagonizing cellular and organism-wide responses to insulin signaling. Cell. 2005;121:115–125. doi: 10.1016/j.cell.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, et al. Caenorhabditis elegans EAK-3 inhibits dauer arrest via nonautonomous regulation of nuclear DAF-16/FOXO activity. Dev Biol. 2008;315:290–302. doi: 10.1016/j.ydbio.2007.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamawaki TM, Arantes-Oliveira N, Berman JR, Zhang P, Kenyon C. Distinct activities of the germline and somatic reproductive tissues in the regulation of Caenorhabditis elegans' longevity. Genetics. 2008;178:513–526. doi: 10.1534/genetics.107.083253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 47.Biddinger SB, Kahn CR. From mice to men: Insights into the insulin resistance syndromes. Annu Rev Physiol. 2006;68:123–158. doi: 10.1146/annurev.physiol.68.040104.124723. [DOI] [PubMed] [Google Scholar]
- 48.Arquier N, et al. Drosophila ALS regulates growth and metabolism through functional interaction with insulin-like peptides. Cell Metab. 2008;7:333–338. doi: 10.1016/j.cmet.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 49.Honegger B, et al. Imp-L2, a putative homolog of vertebrate IGF-binding protein 7, counteracts insulin signaling in Drosophila and is essential for starvation resistance. J Biol. 2008;7:10. doi: 10.1186/jbiol72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Géminard C, et al. Control of metabolism and growth through insulin-like peptides in Drosophila. Diabetes. 2006;55(Suppl. 2):S5–S8. [Google Scholar]
- 51.Boisclair YR, Rhoads RP, Ueki I, Wang J, Ooi GT. The acid-labile subunit (ALS) of the 150 kDa IGF-binding protein complex: An important but forgotten component of the circulating IGF system. J Endocrinol. 2001;170:63–70. doi: 10.1677/joe.0.1700063. [DOI] [PubMed] [Google Scholar]
- 52.Colombani J, et al. A nutrient sensor mechanism controls Drosophila growth. Cell. 2003;114:739–749. doi: 10.1016/s0092-8674(03)00713-x. [DOI] [PubMed] [Google Scholar]
- 53.Zheng X, Yang Z, Yue Z, Alvarez JD, Sehgal A. FOXO and insulin signaling regulate sensitivity of the circadian clock to oxidative stress. Proc Natl Acad Sci USA. 2007;104:15899–15904. doi: 10.1073/pnas.0701599104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garbe JC, Yang E, Fristrom JW. Imp-L2: An essential secreted immunoglobulin family member implicated in neural and ectodermal development in Drosophila. Development. 1993;119:1237–1250. doi: 10.1242/dev.119.4.1237. [DOI] [PubMed] [Google Scholar]
- 55.Andersen AS, Hansen PH, Schaffer L, Kristensen C. A new secreted insect protein belonging to the immunoglobulin superfamily binds insulin and related peptides and inhibits their activities. J Biol Chem. 2000;275:16948–16953. doi: 10.1074/jbc.M001578200. [DOI] [PubMed] [Google Scholar]
- 56.Greer EL, et al. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr Biol. 2007;17:1646–1656. doi: 10.1016/j.cub.2007.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tritos NA, Mantzoros CS. Syndromes of severe insulin resistance. J Clin Endocrinol Metab. 1998;83:3025–3030. doi: 10.1210/jcem.83.9.5143. [DOI] [PubMed] [Google Scholar]
- 58.Hojlund K, et al. A novel syndrome of autosomal-dominant hyperinsulinemic hypoglycemia linked to a mutation in the human insulin receptor gene. Diabetes. 2004;53:1592–1598. doi: 10.2337/diabetes.53.6.1592. [DOI] [PubMed] [Google Scholar]
- 59.Luong N, et al. Activated FOXO-mediated insulin resistance is blocked by reduction of TOR activity. Cell Metab. 2006;4:133–142. doi: 10.1016/j.cmet.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 60.Taguchi A, Wartschow LM, White MF. Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science. 2007;317:369–372. doi: 10.1126/science.1142179. [DOI] [PubMed] [Google Scholar]
- 61.Narbonne P, Roy R. Regulation of germline stem cell proliferation downstream of nutrient sensing. Cell Div. 2006;1:29–38. doi: 10.1186/1747-1028-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.LaFever L, Drummond-Barbosa D. Direct control of germline stem cell division and cyst growth by neural insulin in Drosophila. Science. 2005;309:1071–1073. doi: 10.1126/science.1111410. [DOI] [PubMed] [Google Scholar]
- 63.Hsu HJ, LaFever L, Drummond-Barbosa D. Diet controls normal and tumorous germline stem cells via insulin-dependent and -independent mechanisms in Drosophila. Dev Biol. 2008;313:700–712. doi: 10.1016/j.ydbio.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bruning JC, et al. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- 65.El-Bakri NK, et al. Ovariectomy and gonadal hormone treatment: Effects on insulin-like growth factor-1 receptors in the rat brain. Growth Horm IGF Res. 2004;14:388–393. doi: 10.1016/j.ghir.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 66.Gerisch B, et al. A bile acid-like steroid modulates Caenorhabditis elegans lifespan through nuclear receptor signaling. Proc Natl Acad Sci USA. 2007;104:5014–5019. doi: 10.1073/pnas.0700847104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cargill SL, Carey JR, Muller H-G, Anderson G. Age of ovary determines remaining life expectancy in old ovariectomized mice. Aging Cell. 2003;2:185–190. doi: 10.1046/j.1474-9728.2003.00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tatar M, Chien SA, Priest NK. Negligible senescence during reproductive dormancy in Drosophila melanogaster. Am Nat. 2001;158:248–258. doi: 10.1086/321320. [DOI] [PubMed] [Google Scholar]
- 69.Parmar MKB, Machin D. Survival Analysis: A Practical Approach. Chichester, UK: Wiley; 1995. [Google Scholar]
- 70.Sall J, Lehman A. JMP Start Statistics: A Guide to Statistics and Data Analysis Using JMP and JMP IN Software. Duxbury Press, Belmont: SAS Institute Inc; 1996. [Google Scholar]
- 71.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 72.Cao C, Brown MR. Localization of an insulin-like peptide in brains of two flies. Cell Tiss Res. 2001;304:317–321. doi: 10.1007/s004410100367. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.