Abstract
N-acetylglucosaminyltransferase V (GnT-V) is an enzyme that catalyses β1–6 branching of N-acetylglucosamine on asparagine-linked oligosaccharides of cell proteins. The present study aimed to investigate GnT-V expression and its prognostic significance in endometrial cancer. N-acetylglucosaminyltransferase V expression was studied by immunohistochemistry in 74 surgically resected endometrial cancers, and the staining intensity was evaluated. High GnT-V expression in tumour cells was found in 43 (58.1%) of the 74 cases, and was positively correlated with advanced patient age, histological grade, and lymph vascular space involvement. Patients with high GnT-V expression had significantly impaired overall survival and progression-free survival (PFS) (P=0.0041 and P=0.0023, respectively) compared to patients with low expression of GnT-V. On multivariate analysis, GnT-V expression was an independent prognostic factor for PFS (P=0.0364). β1–6 branching of asparagine-linked oligosaccharides was also detected in GnT-V-positive endometrial cancer cells by leukoagglutinating phytohaemagglutinin (L4-PHA) staining, and the molecular size of the major glycoproteins recognised by L4-PHA was approximately 60–200 kDa by lectin blot analysis. These results suggested that high GnT-V expression was correlated with an unfavourable clinical outcome, and that GnT-V is involved in the malignant potential of endometrial cancer by increasing the synthesis of β1–6 branching of asparagine-linked oligosaccharides.
Keywords: N-acetylglucosaminyltransferase V, endometrial cancer, prognostic factor, progression-free survival (PFS)
The glycosylation of cell-surface glycoproteins is widely accepted to play a key role in a variety of specific biological interactions (Hakomori, 1989). In particular, branching of asparagine-linked oligosaccharides is shown to regulate metastatic potential in cancer cells (Pierce et al, 1997). Among the several patterns of branching, β1–6 branching of N-acetylglucosamine to α-D-6 mannnoside enhances metastasis in experimental cancer models of mice (Dennis et al, 1987). N-acetylglucosaminyltransferase V (GnT-V, EC 4.1.15) catalyses this branching and is most strongly linked to tumour invasion and metastasis. In human cancers, several studies have shown that high activity or expression of GnT-V was associated with poor prognosis in human colorectal cancer (Murata et al, 2000) and breast cancer (Fernandes et al, 1991). On the other hand, GnT-V is expressed in normal human lung and low expression of GnT-V in non-small cell lung cancer is associated with poor prognosis (Dosaka-Akita et al, 2004). Thus, GnT-V expression and its functional and prognostic significance in human cancer remain controversial.
Endometrial cancer is currently the most common gynaecologic malignancy in industrialised countries (Amant et al, 2005). Although this neoplasm is generally considered non-aggressive, it is a heterogeneous disease with 5-year survival rates ranging from over 80–90% for women with clinical stage I disease (Creutzberg et al, 2004). Currently, various clinicopathologic parameters are used to predict the outcome of the disease and to decide the need for adjuvant treatment: surgical stage, histological type, grade, depth of myometrial invasion, cervical stromal invasion, lymph node metastasis, lymph vascular involvement, and peritoneal cytology (Morrow et al, 1991; Grigsby et al, 1992); however, the majority of parameters have been criticised for their subjectivity and poor reproducibility (Nordstrom et al, 1996). Thus, in addition to the conventional clinicopathological parameters, the identification of biochemical or molecular markers more strictly related to the intrinsic biological behaviour of endometrial cancer, and the individualisation of adjuvant therapy based on more reliable prognostic indicators, may be helpful to further improve the survival of patients, as well as to prevent the unnecessary use of adjuvant therapy.
In the present study, we examined GnT-V expression by immunohistochemistry in surgically resected endometrial cancer and analysed its biological and clinical importance, especially as a potential prognostic factor.
MATERIALS AND METHODS
Patients and tissue specimens
Seventy-four patients with endometrial endometrioid adenocarcinoma between 1990 and 2005 were included in this study. Initial diagnoses were made preoperatively by the pathological review of endometrial biopsy or curettage specimens. Surgical treatment consisted of total abdominal hysterectomy and bilateral salpingooophorectomy, followed by surgical staging, including peritoneal washing cytology and lymphadenectomy. Patients with histological cell types other than endometrioid adenocarcinoma, such as papillary serous or clear cell, were not included in this study. The mean age of the patients was 58.3 years (range: 38–86). All patients were staged according to the 1988 International Federation of Gynecology and Obstetrics (FIGO) criteria: 43 were stage I (four were IA, 29 were IB, 10 were IC), nine were stage II, 17 were stage III, and five were stage IV. Histological grade was assigned according to the criteria of the World Health Organisation (WHO) classification: 28 were G1 (well differentiated), 33 were G2 (moderately differentiated), and 13 were G3 (poorly differentiated). In this study, all patients with FIGO stage IC and more advanced-stage disease received post-operative adjuvant chemotherapy with six cycles of cisplatin/doxorubicin/cyclophosphamide or cisplatin plus etoposide in 1992–1999, and carboplatin plus paclitaxel after 2000. Patients receiving post-operative radiation therapy or any preoperative treatment were excluded from this study because the number of patients was very small. Tumour recurrence/progression was defined based on clinical, radiological, or histological diagnosis. Patients with recurrence were treated with chemotherapy, local radiation therapy, or surgical tumour resection if possible.
Western blot analysis
JAR human choriocarcinoma cells were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA) and UtSMC normal human uterine smooth muscle cell line (CC-2562) from Cambrex (Walkersville, MD, USA). Tumour tissue samples and cells were homogenised in a lysis buffer consisting of a protease inhibitor mixture in radioimmunoprecipitation assay buffer. After centrifugation at 15 000 g for 20 min, the supernatant was obtained. Twenty micrograms of protein extract was separated by SDS 10% polyacrylamide gel electrophoresis, transferred onto a nitrocellulose membrane, and immunoblotted with anti-GnT-V monoclonal antibody (24D11) (Nakahara et al, 2003) at a dilution of 1 : 1000. Immunoreactive proteins were stained using a chemiluminescence detection system (ECL, GE Healthcare, Buckinghamshire, UK).
Leukoagglutinating phytohaemagglutinin blot analysis
Protein-blotted nitrocellulose filters were prepared in exactly the same way as described for Western blotting. After blocking with 5% skim milk for 30 min at room temperature, the filter was incubated in PBS containing 1 : 1000 diluted biotinylated leukoagglutinating phytohaemagglutinin (L4-PHA, Seikagaku, Tokyo, Japan), which preferentially recognises β1–6 branches of tri- or tetra-antennary sugar chains, for 1 h at room temperature. The filter was washed three times with PBS containing 0.05% Tween 20 (TPBS) for 10 min each. Substrate binding was detected with a 1 : 1000 dilution of avidin–peroxidase conjugate (ABC kit, Vector Res., CA, USA) in TPBS for 30 min at room temperature. The membrane was washed and then developed using ECL reagents (GE Healthcare, Buckinghamshire, UK).
Lectin blot analysis on immunoprecipitated β1 integrin
For immunoprecipitation, 800 μg of proteins were extracted from each sample tissue. After incubation with 1 μl of anti-human β1 integrin mAb MAB2247 (Chemicon International Inc., Temecula, CA, USA) overnight at 4°C, immune complexes were collected with 30 μl of protein G-Sepharose 4EF beads (GE Healthcare, Buckinghamshire, UK). The complexes were released by boiling in sampling buffer without a detergent, separated by 7.5% SDS–PAGE. The membrane filter was analysed by L4-PHA lectin blot, as described above. After deprobing and blocking, it was subjected to Western blot analysis using anti-β1 integrin mAb as described above.
Immunohistochemistry for GnT-V
Informed consent was obtained from individual patients for the use of their tissue samples. A mouse monoclonal antibody against recombinant human GnT-V was made according to the standard protocol, as described previously (Murata et al, 2000). Briefly, mice were immunised by recombinant GnT-V, and monoclonal antibodies for GnT-V were screened by the availability for immunohistochemistry. The antibody used in the present study recognised 545SKNTDFFIGKPTILRELTS562 of the human GnT-V amino-acid sequence and gave the best signal for immunohistochemistry. Surgical specimens were fixed in 10% formalin and embedded in paraffin. Paraffin specimens were cut at a thickness of 4 μm. For heat-induced epitope retrieval, deparaffinised sections were soaked in Target Retrieval Solution consisting of 10 mM Tris and 1 mM EDTA (DAKO, Glostrup, Denmark), and treated at 95°C for 30 min in a microwave oven. Immunohistochemical staining was performed using the avidin–biotin immunoperoxidase technique (Histofine SAB-PO kit, Nichirei, Tokyo, Japan). Endogenous peroxidase activity was blocked by incubation with 0.3% H2O2 in methanol for 15 min, and nonspecific immunoglobulin binding was blocked by incubation with 10% normal goat serum for 10 min. Sections were incubated at 4°C overnight with anti-GnT-V antibody at 1 : 400 dilution or antiproliferating cell nuclear antigen (PCNA) antibody (DAKO) at 1 : 30. The sections were rinsed and incubated for 30 min with the biotinylated second antibody. After washing, the sections were incubated for 5 min with horseradish peroxidase-conjugated streptavidin, and finally treated with 3,3′-diaminobenzidine tetrahydrochloride (Nichirei, Tokyo, Japan) in 0.01% H2O2 for 3 min. The slides were counterstained with Meyer's haematoxylin. As a negative control, the primary antibody was replaced with normal mouse IgG at an appropriate dilution. As a positive control, tissue sections of normal placenta were used as reported previously (Tomiie et al, 2005). N-acetylglucosaminyltransferase V expression levels were classified semiquantitatively based on the total scores of the per cent positivity of stained tumour cells and the staining intensity. Namely, the per cent positivity was scored as ‘0’ if <5% (negative), ‘1’ if 5–30% (sporadic), ‘2’ if 30–70% (focal), and ‘3’ if >70% (diffuse) of cells stained, whereas staining intensity was scored relative to the known positive and negative controls as ‘0’ if no staining, ‘1’ if weakly stained, ‘2’ if moderately stained (intermediate level between strong and weak), and ‘3’ if strongly stained. The final GnT-V expression score was defined as follows: ‘GnT-V low’ if the sum of the per cent positivity score and the staining intensity score was 0–4 and ‘GnT-V high’ if the sum was 5–6. In each case, at least three different areas were evaluated. The scoring procedure was carried out twice by two independent observers without any knowledge of the clinical data. The concordance rate was over 95% between the observers. In case of disagreement, the slides were reviewed simultaneously by these two observers, together with another observer, who were seated together at a multiheaded microscope, to resolve the difference of opinion.
Leukoagglutinating phytohaemagglutinin histochemistry
The expression of β1–6 branching asparagine-linked oligosaccharides was analysed by L4-PHA histochemistry, with a modified previous method (Suzuki et al, 1999). Briefly, after deparaffinisation, trypsinisation was performed in Tris buffer containing 0.1% trypsin (Difco Laboratories, Detroit, MI, USA) and 0.1% CaCl2 for 10 min at 37°C after blocking endogenous peroxidase activity. The sections were incubated with 5% skim milk in PBS for 20 min at room temperature to block nonspecific staining. The sections were incubated with HRP-PHA-L4 (Seikagaku) at a dilution of 1 : 200 at 4°C overnight. Staining was performed by the biotin–streptavidin peroxidase method with 3,3′-diaminobenzidine as a chromogen. Haematoxylin was used as a counterstain.
Statistical analysis
Statistical analysis was performed using χ2 for the independence test, Fisher's exact probability test, or Student's t-test. For survival analysis, the Kaplan–Meier method was applied, and statistical significance was calculated using the log-rank test. Cox proportional-hazard analysis was used for univariate and multivariate analyses to explore the effect of variables on survival. StatView software ver.5.0 (SAS Institute Inc., Cary, NC, USA) was used for statistical analyses, and a P-value of <0.05 was considered significant.
RESULTS
GnT-V protein expression and lectin blot analysis in endometrial cancer tissue
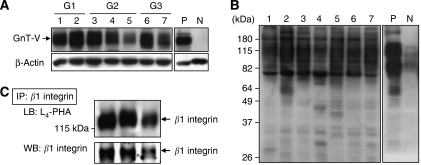
First, the GnT-V protein expression was examined in endometrial cancer tissues obtained from seven patients using Western blot analysis. In all samples, GnT-V protein was detected as approximately 110 kDa bands, although its expression level varied among the samples (Figure 1A). The broad bands of GnT-V may be due to oligosaccharide modification of GnT-V, because GnT-V has as many as five sugar chains and the molecular weight of GnT-V is smaller with less β1–6GlcNAc branching (Nakahara et al, 2003).
Figure 1.
GnT-V and β1–6 branching glycoprotein expression, and β1–6GlcNAc branching of β1 integrin in endometrial cancer tissues. (A) Western blot analysis with anti-GnT-V mAb. (B) Lectin blot analysis with L4-PHA. Lanes 1–7 corresponded to seven different endometrial cancer patients (G1, grade 1; G2, grade 2; G3, grade 3). P, JAR is a choriocarcinoma cell line used as a positive control for GnT-V expression; N, normal human uterine smooth muscle cells (UtSMC) as a negative control. (C) β1 integrin was immunoprecipitated from endometrial cancer tissues from three different patients. The amount of β1–6GlcNAc branching of β1 integrin was analysed by means of an L4-PHA lectin blot (upper panel). The membrane was reprobed with a specific mAb to β1 integrin (lower panel).
To evaluate the level of β1–6 branching, we also performed lectin blot analysis on total cellular proteins using L4-PHA, which preferentially binds to GlcNAc residues on β1–6 branches of tri- or tetra-antennary sugar chains (Figure 1B). This analysis showed that GnT-V certainly catalysed such specific glycosylation to target glycoproteins, whose major molecular sizes were approximately 60–200 kDa.
Lectin blot analysis on immunoprecipitated β1 integrin
It has been reported that β1 integrin is a target molecule of GnT-V in certain cell lines (Guo et al, 2002; Nakahara et al, 2003); therefore, to investigate the glycosylation state of β1 integrin, we performed L4-PHA blot analysis on immunoprecipitated β1 integrin. The results showed that β1 integrin, which had been immunoprecipitated from endometrial cancer tissues, certainly contained β1–6 GlcNAc branching (Figure 1C), suggesting that β1 integrin is a target substrate of GnT-V in endometrial cancers.
Immunohistochemical expression of GnT-V and L4-PHA staining in endometrial cancer tissues
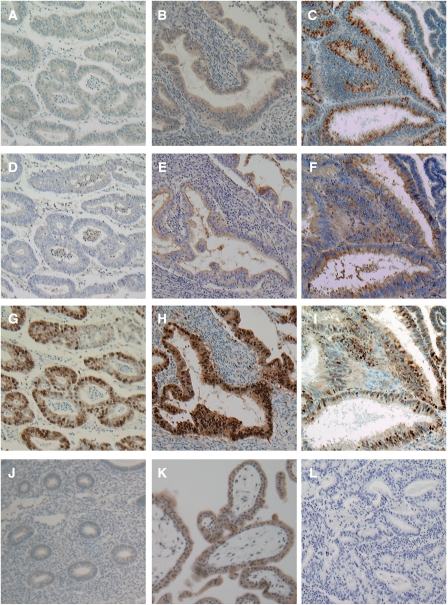
We examined GnT-V expression in endometrial cancer tissues by immunohistochemical staining using 74 surgical specimens. As shown in Figure 2A–C, GnT-V immunoreactivity was detected at variable levels, and was found in the cytoplasm of cancer cells, which were identified using PCNA co-staining (Figure 2G–I). In contrast, GnT-V immunoreactivity was very faint or absent in tumour stroma (Figure 2A–C) and normal endometrium (Figure 2J). N-acetylglucosaminyltransferase V immunoreactivity was not detected in the negative control experiment (Figure 2L), whereas it was strongly detected in placental tissues used as a positive control (Figure 2K). Next, we examined the expression of β1–6 branching asparagine-linked oligosaccharides by L4-PHA histochemistry in the same sections, simultaneously. Leukoagglutinating phytohaemagglutinin staining was very weak in tumour cells that showed weak GnT-V immunostaining (Figure 2D), while it was moderate-to-strong in cancer cells that showed high GnT-V immunostaining (Figure 2E and F). These results were consistent with the results of lectin blotting, which recognised β1–6 branching in endometrial cancer with variable intensity.
Figure 2.
Immunohistochemical staining patterns for GnT-V and staining of L4-PHA in endometrial cancers. Staining pattern of a tumour: (A) GnT-V low; (B and C) GnT-V high. (D–F) L4-PHA staining and (G–I) PCNA immunostaining were performed simultaneously with the same A, B, and C specimens, respectively. (J) Normal endometrial cells showed very faint or negative GnT-V expression. (K) Positive control for GnT-V (normal placenta). (L) Negative control. Original magnification, × 100.
Correlation of GnT-V expression with clinicopathological parameters
Of the 74 specimens examined, ‘low GnT-V expression’ tumours were found in 31 (41.9%) cases, and ‘high GnT-V expression’ in 43 (58.1%) cases, respectively. The correlations of high GnT-V expression with various clinicopathological parameters in the 74 cases are summarised in Table 1. High GnT-V expression was positively correlated with age (P=0.045), histological grade (P=0.011), and lymph vascular space involvement (P<0.001), but not with the FIGO surgical stage, lymph node metastasis, and myometrial invasion.
Table 1. Correlation of GnT-V expression with clinicopathological factors in endometrial cancer.
|
GnT-V expression
|
||||
|---|---|---|---|---|
| Characteristics | Patient no. | Low | High | P-valuea |
| All cases | 74 | 31 (41.9%) | 43 (58.1%) | |
| Age | ||||
| ⩽60 | 45 | 23 (51.1%) | 22 (48.9%) | 0.045 |
| >60 | 29 | 8 (27.6%) | 21 (72.4%) | |
| FIGO surgical stage | ||||
| I+II | 52 | 25 (48.1%) | 27 (51.9%) | 0.097 |
| III+IV | 22 | 6 (27.3%) | 16 (72.3%) | |
| Histological grading | ||||
| G1 | 28 | 17 (60.7%) | 11 (39.3%) | 0.011 |
| G2/G3 | 46 | 14 (30.4%) | 32 (69.6%) | |
| Lymph vascular invasion | ||||
| Negative | 40 | 24 (60.0%) | 16 (40.0%) | <0.001 |
| Positive | 34 | 7 (20.6%) | 27 (79.4%) | |
| Nodal status | ||||
| N0 | 60 | 27 (45.0%) | 33 (55.0%) | 0.262 |
| N1 | 14 | 4 (28.6%) | 10 (71.4%) | |
| Myometrial invasion | ||||
| 1/2> | 43 | 20 (46.5%) | 23 (53.5%) | 0.343 |
| 1/2⩽ | 31 | 11 (35.5%) | 20 (64.5%) | |
Abbreviations: FIGO=International Federation of Gynecology and Obstetrics; GnT-V=N-acetylglucosaminyltransferase V.
χ2-test.
Correlation of GnT-V expression with patient survival
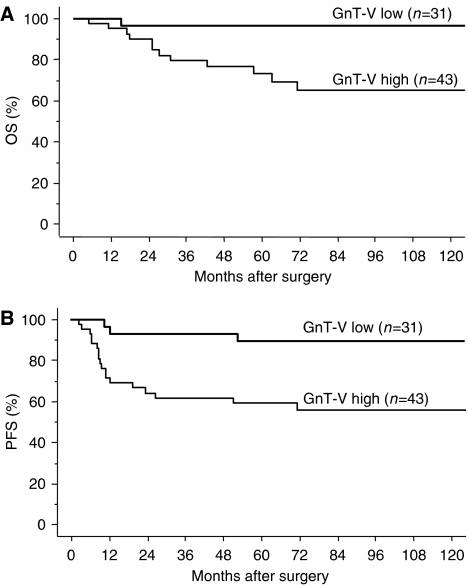
Follow-up data were available for all 74 patients and the median follow-up period was 72.9 months (range: 3–160). During the follow-up period, disease progression/recurrence was observed in 20 patients (27.0%), of which 12 (16.2%) died. The median time to progression/recurrence and death was 15.0 and 28.5 months, respectively. To evaluate the impact of GnT-V expression on patient prognosis, overall survival (OS) and progression-free survival (PFS) curves were constructed using the Kaplan–Meier method. The OS rates of patients with GnT-V low and GnT-V high were 96.8 and 74.4%, respectively (Figure 3A). The 5-year PFS rates for GnT-V low and GnT-V high were 90.3 and 60.5%, respectively (Figure 3B). Patients with high GnT-V expression had significantly impaired OS (P=0.0041) and PFS (P=0.0023) as compared to patients with low expression of GnT-V (Figure 3A and B).
Figure 3.
Overall survival (OS) and progression-free survival (PFS) curve drawn using the Kaplan–Meier method according to GnT-V expression in endometrial cancer patients. OS (A) and PFS (B) in all patients (n=74). Significant differences in OS (P=0.0041), and PFS (P=0.0023).
Multivariate analysis of prognostic variables in endometrial cancer patients
Cox proportional-hazard analysis was performed to compare the impact of GnT-V expression on survival with currently used clinicopathological prognostic factors. The results of univariate/multivariate analyses of the variables, including GnT-V expression, age, surgical stage, grade, lymph vascular space involvement, node status, and myometrial invasion, with respect to OS and PFS, are shown in Tables 2 and 3, respectively. Among the seven variables, there was no significant prognostic factor with respect to OS on multivariate analysis, although the surgical stage, lymph vascular invasion, node status, myometrial invasion, and GnT-V expression were significant prognostic factors on univariate analysis (Table 2). In contrast, only GnT-V expression was found to be an independent prognostic factor (hazard ratio=4.164, P=0.0364) with respect to PFS on multivariate analysis (Table 3).
Table 2. Univariate and multivariate analyses of overall survival in endometrial cancer patients.
|
Multivariate analysis
|
|||||
|---|---|---|---|---|---|
| Variables | Categories | Univariate P valuea | HR | 95% CI | P-value |
| Age | ⩽60 | ||||
| >60 | 0.8046 | — | — | — | |
| FIGO surgical stage | I+II | ||||
| III+IV | <0.0001 | 2.922 | 0.639–13.362 | 0.1667 | |
| Histological grading | G1 | ||||
| G2/G3 | 0.1425 | — | — | — | |
| Lymph vascular invasion | Negative | ||||
| Positive | 0.0004 | 1.872 | 0.261–13.446 | 0.5329 | |
| Nodal status | N0 | ||||
| N1 | <0.0001 | 3.466 | 0.940–12.787 | 0.062 | |
| Myometrial invasion | 1/2> | ||||
| 1/2⩽ | 0.011 | 1.236 | 0.292–5.224 | 0.7734 | |
| GnT-V | Low | ||||
| High | 0.0041 | 6.053 | 0.649–56.452 | 0.5329 | |
Abbreviations: CI=confidence interval; FIGO=International Federation of Gynecology and Obstetrics; GnT-V=N-acetylglucosaminyltransferase V; HR=hazard ratio.
Log-rank test.
Table 3. Univariate and multivariate analyses of progression-free survival in endometrial cancer patients.
|
Multivariate analysisa
|
|||||
|---|---|---|---|---|---|
| Variables | Categories | Univariate P valuea | HR | 95% CI | P-value |
| Age | ⩽60 | ||||
| >60 | 0.8643 | — | — | — | |
| FIGO surgical stage | I+II | ||||
| III+IV | <0.0001 | 3.049 | 0.997–9.327 | 0.0507 | |
| Histological grading | G1 | ||||
| G2/G3 | 0.0625 | — | — | — | |
| Lymph vascular invasion | Negative | ||||
| Positive | 0.0003 | 1.352 | 0.350–5.222 | 0.6616 | |
| Nodal status | N0 | ||||
| N1 | <0.0001 | 2.242 | 0.789–6.365 | 0.1295 | |
| Myometrial invasion | 1/2> | ||||
| 1/2⩽ | 0.0048 | 1.386 | 0.441–4.356 | 0.5761 | |
| GnT-V | Low | ||||
| High | 0.0023 | 4.164 | 1.095–15.840 | 0.0364 | |
Abbreviations: CI=confidence interval; FIGO=International Federation of Gynecology and Obstetrics; GnT-V=N-acetylglucosaminyltransferase V; HR=hazard ratio.
Log-rank test.
DISCUSSION
In the present study, we demonstrated the expression of GnT-V in endometrial cancer using 74 surgical specimens, and found that high GnT-V expression by tumour cells was positively correlated with impaired clinical outcome. The immunoreactivity of GnT-V was very weak in normal endometrium and increased clearly in endometrial cancer. Oligosaccharides on glycoproteins are altered in tumorigenesis and often play a role in the regulation of the biological characteristics of tumours (Hakomori, 1989). Each oligosaccharide is synthesised by a specific glycosyltransferase whose expression affects a specific function of glycoprotein through glycosylation in normal and malignant cells (Varki, 1993). Among many glycosyltransferases and oligosaccharides, GnT-V and its products, β1–6 branching N-linked oligosaccharides, have been associated with the malignant potential of cancer (Dennis et al, 1987). In colon cancer, breast cancer, and oesophageal cancer, GnT-V expression has a positive correlation with poor prognosis, which is consistent with our results in the present study (Fernandes et al, 1991; Murata et al, 2000; Ishibashi et al, 2005). On the other hand, low GnT-V expression is associated with shorter survival and poor prognosis in non-small cell lung cancer, bladder cancer, and hepatocellular cancer (Ito et al, 2001; Dosaka-Akita et al, 2004; Ishimura et al, 2006). It may depend on the type of cancer or originating tissues whether GnT-V expression is associated positively with poor prognosis.
We confirmed the levels of β1–6 branching in endometrial cancers using lectin blotting and L4-PHA histochemistry. N-acetylglucosaminyltransferase V expression is not equal to the expression of β1–6 branching asparagine-linked oligosaccharides analysed by L4-PHA histochemistry (Dosaka-Akita et al, 2004). This is because GnT-V has been shown to function as an inducer of angiogenesis (Saito et al, 2002), which is completely different from the original function of glycosyltransferase, and GnT-V expression does not necessarily result in the synthesis of β1–6 branching oligosaccharides. Our results showed that GnT-V-expression intensity was well consistent with L4-PHA-staining intensity in tumour cells. These findings suggested that GnT-V plays a functional role in the malignant potential of endometrial cancer cells by the synthesis of β1–6 branching oligosaccharides.
Our lectin blotting revealed that major target glycoproteins of GnT-V in endometrial cancer were 60–200 kDa in molecular size. Previous reports indicated several specific substrates for GnT-V glycosylation and changes in the biological characteristics of cancer cells. An increased level of β1–6 branching on β1 integrin, a 130 kDa subunit of fibronectin receptor, by GnT-V resulted in the inhibition of cisplatin-induced apoptosis, or inhibition of clustering of α5β1 integrin and promotion of cell migration in neck squamous cell carcinoma and fibrosarcoma (Guo et al, 2002; Nakahara et al, 2003). Lamp-1 is a 90–120 kDa molecule expressed on cell and lysosome membranes, and plays an important role in lysosomal trafficking, matrix degradation, and cell adhesion. N-acetylglucosaminyltransferase V glycosylation of lamp-1 inhibits its degradation, and the stabilisation of lamp-1 results in increased extracellular matrix degradation (Fukuda, 1991; Kundra and Kornfeld, 1999). Matriptase is an 80 kDa serine protease involved in cancer metastasis by the activation of urokinase-type plasminogen activator (u-PA) and hepatocyte growth factor (Lee et al, 2000). The addition of β1–6 branching on matriptase by GnT-V inhibits its degradation, resulting in the upregulation of matriptase expression in gastric cancer (Ihara et al, 2002). Of those molecules, matriptase and β1 integrin were expressed in endometrial cancer, especially β1 integrin with β1–6 branching by GnT-V (Figure 1C). Increased GnT-V did not change the expression of α5β1 integrin, but increased the level of β1–6 branching on it, and subsequently inhibited integrin clustering and signal transduction pathways. As a result, cell migration and invasion were stimulated (Guo et al, 2002; Nakahara et al, 2006). In the present study, we showed that high GnT-V expression was significantly correlated with lymph vascular invasion and the histological grade. These results suggested that GnT-V might be involved in tumour cell migration or invasion by the modification of oligosaccharides of β1 integrin in endometrial cancer, resulting in disease progression and poor prognosis. In addition, GnT-V might be linked to malignant potential, increasing β1–6 branching synthesis in poorly differentiated cancer cells; however, the functional significance of GnT-V expression in endometrial cancer has to be studied further.
In conclusion, we demonstrated that high GnT-V expression correlated with impaired clinical outcome in endometrial cancer patients. Furthermore, GnT-V was an independent prognostic factor for PFS. These results indicate that GnT-V is a reliable and promising prognostic indicator and might become a novel molecular target in the strategy for the treatment of endometrial cancer.
Acknowledgments
This work was supported by Grants-in-aid no.18799005 (to EY) from the Japanese Ministry of Education, Culture, Sports, Science, and Technology.
References
- Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I (2005) Endometrial cancer. Lancet 366: 491–505 [DOI] [PubMed] [Google Scholar]
- Creutzberg CL, van Putten W.L, Warlam-Rodenhuis CC, van den Bergh AC, de Winter KA, Koper PC, Lybeert ML, Slot A, Lutgens LC, Stenfert Kroese MC, Beerman H, van Lent M (2004) Outcome of high-risk stage IC, grade 3, compared with stage I endometrial carcinoma patients: the Postoperative Radiation Therapy in Endometrial Carcinoma Trial. J Clin Oncol 22: 1234–1241 [DOI] [PubMed] [Google Scholar]
- Dennis JW, Laferte S, Waghorne C, Breitman ML, Kerbel RS (1987) Beta 1–6 branching of Asn-linked oligosaccharides is directly associated with metastasis. Science 236: 582–585 [DOI] [PubMed] [Google Scholar]
- Dosaka-Akita H, Miyoshi E, Suzuki O, Itoh T, Katoh H, Taniguchi N (2004) Expression of N-acetylglucosaminyltransferase v is associated with prognosis and histology in non-small cell lung cancers. Clin Cancer Res 10: 1773–1779 [DOI] [PubMed] [Google Scholar]
- Fernandes B, Sagman U, Auger M, Demetrio M, Dennis JW (1991) Beta 1–6 branched oligosaccharides as a marker of tumor progression in human breast and colon neoplasia. Cancer Res 51: 718–723 [PubMed] [Google Scholar]
- Fukuda M (1991) Lysosomal membrane glycoproteins. Structure, biosynthesis, and intracellular trafficking. J Biol Chem 266: 21327–21330 [PubMed] [Google Scholar]
- Grigsby PW, Perez CA, Kuten A, Simpson JR, Garcia DM, Camel HM, Kao MS, Galakatos AE (1992) Clinical stage I endometrial cancer: prognostic factors for local control and distant metastasis and implications of the new FIGO surgical staging system. Int J Radiat Oncol Biol Phys 22: 905–911 [DOI] [PubMed] [Google Scholar]
- Guo HB, Lee I, Kamar M, Akiyama SK, Pierce M (2002) Aberrant N-glycosylation of beta1 integrin causes reduced alpha5beta1 integrin clustering and stimulates cell migration. Cancer Res 62: 6837–6845 [PubMed] [Google Scholar]
- Hakomori S (1989) Aberrant glycosylation in tumors and tumor-associated carbohydrate antigens. Adv Cancer Res 52: 257–331 [DOI] [PubMed] [Google Scholar]
- Ihara S, Miyoshi E, Ko JH, Murata K, Nakahara S, Honke K, Dickson RB, Lin CY, Taniguchi N (2002) Prometastatic effect of N-acetylglucosaminyltransferase V is due to modification and stabilization of active matriptase by adding beta 1–6 GlcNAc branching. J Biol Chem 277: 16960–16967 [DOI] [PubMed] [Google Scholar]
- Ishibashi Y, Dosaka-Akita H, Miyoshi E, Shindoh M, Miyamoto M, Kinoshita I, Miyazaki H, Itoh T, Kondo S, Nishimura M, Taniguchi N (2005) Expression of N-acetylglucosaminyltransferase V in the development of human esophageal cancers: immunohistochemical data from carcinomas and nearby noncancerous lesions. Oncology 69: 301–310 [DOI] [PubMed] [Google Scholar]
- Ishimura H, Takahashi T, Nakagawa H, Nishimura S, Arai Y, Horikawa Y, Habuchi T, Miyoshi E, Kyan A, Hagisawa S, Ohyama C (2006) N-acetylglucosaminyltransferase V and beta1–6 branching N-linked oligosaccharides are associated with good prognosis of patients with bladder cancer. Clin Cancer Res 12: 2506–2511 [DOI] [PubMed] [Google Scholar]
- Ito Y, Miyoshi E, Sakon M, Takeda T, Noda K, Tsujimoto M, Ito S, Honda H, Takemura F, Wakasa K, Monden M, Matsuura N, Taniguchi N (2001) Elevated expression of UDP-N-acetylglucosamine: alphamannoside beta1,6 N-acetylglucosaminyltransferase is an early event in hepatocarcinogenesis. Int J Cancer 91: 631–637 [PubMed] [Google Scholar]
- Kundra R, Kornfeld S (1999) Asparagine-linked oligosaccharides protect Lamp-1 and Lamp-2 from intracellular proteolysis. J Biol Chem 274: 31039–31046 [DOI] [PubMed] [Google Scholar]
- Lee SL, Dickson RB, Lin CY (2000) Activation of hepatocyte growth factor and urokinase/plasminogen activator by matriptase, an epithelial membrane serine protease. J Biol Chem 275: 36720–36725 [DOI] [PubMed] [Google Scholar]
- Morrow CP, Bundy BN, Kurman RJ, Creasman WT, Heller P, Homesley HD, Graham JE (1991) Relationship between surgical–pathological risk factors and outcome in clinical stage I and II carcinoma of the endometrium: a Gynecologic Oncology Group study. Gynecol Oncol 40: 55–65 [DOI] [PubMed] [Google Scholar]
- Murata K, Miyoshi E, Kameyama M, Ishikawa O, Kabuto T, Sasaki Y, Hiratsuka M, Ohigashi H, Ishiguro S, Ito S, Honda H, Takemura F, Taniguchi N, Imaoka S (2000) Expression of N-acetylglucosaminyltransferase V in colorectal cancer correlates with metastasis and poor prognosis. Clin Cancer Res 6: 1772–1777 [PubMed] [Google Scholar]
- Nakahara S, Miyoshi E, Noda K, Ihara S, Gu J, Honke K, Inohara H, Kubo T, Taniguchi N (2003) Involvement of oligosaccharide changes in alpha5beta1 integrin in a cisplatin-resistant human squamous cell carcinoma cell line. Mol Cancer Ther 2: 1207–1214 [PubMed] [Google Scholar]
- Nakahara S, Saito T, Kondo N, Moriwaki K, Noda K, Ihara S, Takahashi M, Ide Y, Gu J, Inohara H, Katayama T, Tohyama M, Kubo T, Taniguchi N, Miyoshi E (2006) A secreted type of beta1,6 N-acetylglucosaminyltransferase V (GnT-V), a novel angiogenesis inducer, is regulated by gamma-secretase. FASEB J 20: 2451–2459 [DOI] [PubMed] [Google Scholar]
- Nordstrom B, Strang P, Lindgren A, Bergstrom R, Tribukait B (1996) Carcinoma of the endometrium: do the nuclear grade and DNA ploidy provide more prognostic information than do the FIGO and WHO classifications? Int J Gynecol Pathol 15: 191–201 [DOI] [PubMed] [Google Scholar]
- Pierce M, Buckhaults P, Chen L, Fregien N (1997) Regulation of N-acetylglucosaminyltransferase V and Asn-linked oligosaccharide beta(1,6) branching by a growth factor signaling pathway and effects on cell adhesion and metastatic potential. Glycoconj J 14: 623–630 [DOI] [PubMed] [Google Scholar]
- Saito T, Miyoshi E, Sasai K, Nakano N, Eguchi H, Honke K, Taniguchi N (2002) A secreted type of beta 1,6-N-acetylglucosaminyltransferase V (GnT-V) induces tumor angiogenesis without mediation of glycosylation: a novel function of GnT-V distinct from the original glycosyltransferase activity. J Biol Chem 277: 17002–17008 [DOI] [PubMed] [Google Scholar]
- Suzuki O, Nozawa Y, Kawaguchi T, Abe M (1999) Phaseolus vulgaris leukoagglutinating lectin-binding reactivity in human diffuse large B-cell lymphoma and its relevance to the patient's clinical outcome: lectin histochemistry and lectin blot analysis. Pathol Int 49: 874–880 [DOI] [PubMed] [Google Scholar]
- Tomiie M, Isaka S, Miyoshi E, Taniguchi N, Kimura T, Ogita K, Tsutsui T, Shimoya K, Nakagawa T, Kondo A, Koyama M, Murata Y (2005) Elevated expression of N-acetylglucosaminyltransferase V in first trimester human placenta. Biochem Biophys Res Commun 330: 999–1004 [DOI] [PubMed] [Google Scholar]
- Varki A (1993) Biological roles of oligosaccharides: all of the theories are correct. Glycobiology 3: 97–130 [DOI] [PMC free article] [PubMed] [Google Scholar]