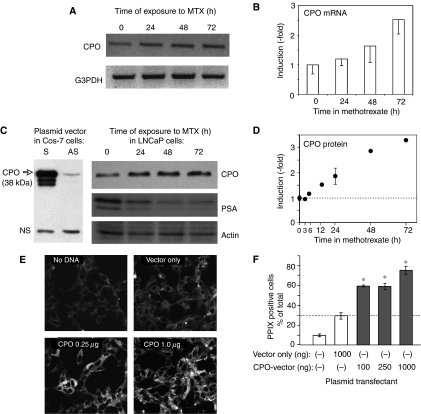
Figure 7.
Demonstration that MTX treatment leads to increased levels of CPO. (A) Methotrexate treatment of LNCaP cells causes accumulation of CPO mRNA. Cells were exposed for various times to MTX (1 mg l−1), or remained untreated (0 h). Cells were lysed, cDNA prepared, and RT–PCR performed using a CPO gene-specific primer set, as described in Materials and Methods. The resulting PCR product was confirmed by DNA sequencing and by size-similarity to a known plasmid cDNA (not shown). The ethidium-stained, CPO-specific bands are shown here inverted (dark bands). G3PDH, invariant control. (B) Semiquantitative analysis of CPO mRNA. Agarose gels were digitally photographed, and the integrated density of each band measured using NIH Image software. Induction (relative to untreated controls) was calculated after background subtraction and normalisation to the G3PDH bands. Mean±range of two experiments. (C, left side) The anti-CPO epitope antibody recognises full-length CPO protein. Lysates from cos-7 cells were transfected with a plasmid vector (pCPO) expressing CPO inserted in either the sense (S) or antisense (AS) orientation, and analysed by Western blot using the antiserum to CPO. Arrow: CPO-specific signal; NS, nonspecific. (C, right side) CPO is expressed in LNCaP cells and increases with MTX treatment. Equal amounts of protein from lysed cells were separated on acrylamide gels, and immunoblotted with antibodies to CPO, prostate-specific antigen, or actin. The 38 kDa CPO protein is indicated. (D) Semiquantitative analysis of CPO protein. Western blots from experiments examining long and short times of exposure to MTX were scanned densitometrically, and the data combined. Error bars: range of duplicate experiments. (E) Fluorescence images, captured from LNCaP cells that were either not transfected (No DNA), transfected with an empty pcDNA3.1 vector (Vector only), or transfected with different amounts of a plasmid vector overexpressing CPO, followed by incubation with ALA and analysis of the PpIX-specific signal by confocal microscopy. The PpIX-specific signal is shown in grey scale. (F) Semiquantitative analysis of relative PpIX-positive cells (% of total cells) was performed as described in Materials and Methods. Bars represent the mean±s.d. of three images from each of two dishes; Asterisks, P<0.0005 relative to the vector-only control.