Fig. 3.
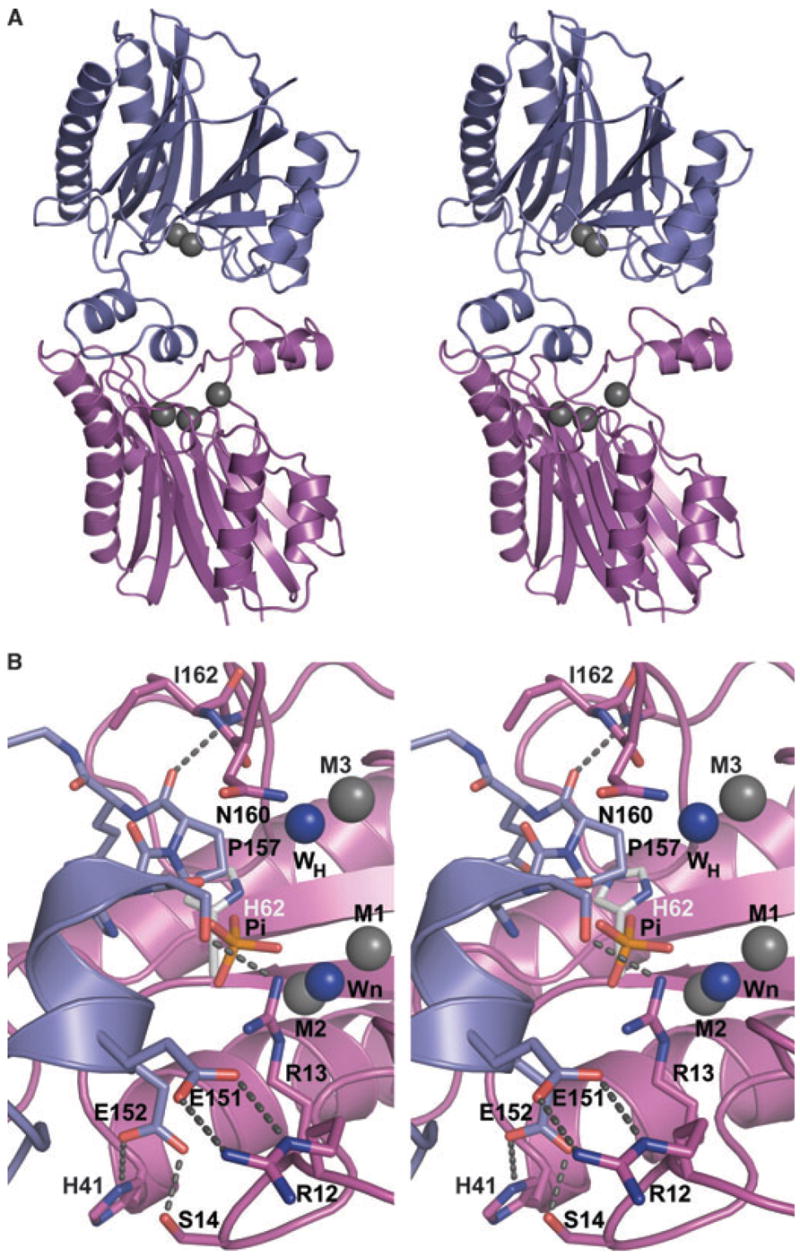
(A) Stereoimage showing the packing interactions between selected monomers (A and C) that mimics substrate binding. Monomer C (magenta) binds a peptide from the loop of monomer A (blue) to the active site. Metal ions are shown as gray spheres. Notably, monomer A binds two metals, whereas monomer C binds three. (B) Closeup view of crystal contact indicating the potential position of phosphate based on HsSTP. Coloring is as in (A) except that the nucleophilic (Wn) and putative general acid (WH) water molecules are shown as blue spheres, and residues participating in the interactions are shown as sticks. The direct interactions across the interface are indicated by dashed lines. The phosphate found in HsSTP was modeled into SaSTP by aligning the HsSTP and SaSTP monomers active sites. In the alignment metal ions M1 and M2, Wn, and carboxylate groups of Asp36, Asp192 and Asp231 were used. Alignment of 12 atoms resulted in an rmsd value of 0.3 Å. As shown, the modeled position of the phosphate from HsSTP is close to Ser155(A) and Arg13(C). Also, the general acid in HsSTP, His62, is shown as sticks with gray carbon atoms.
