Abstract
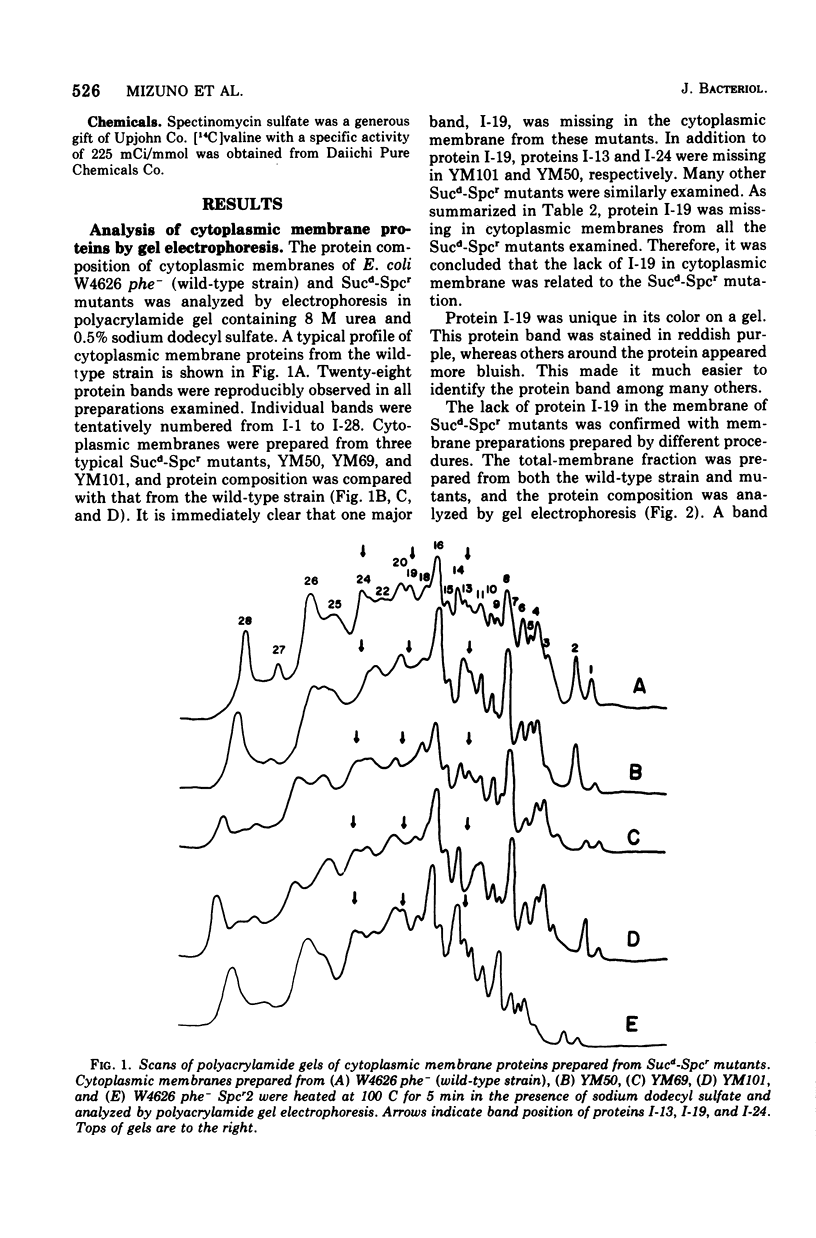
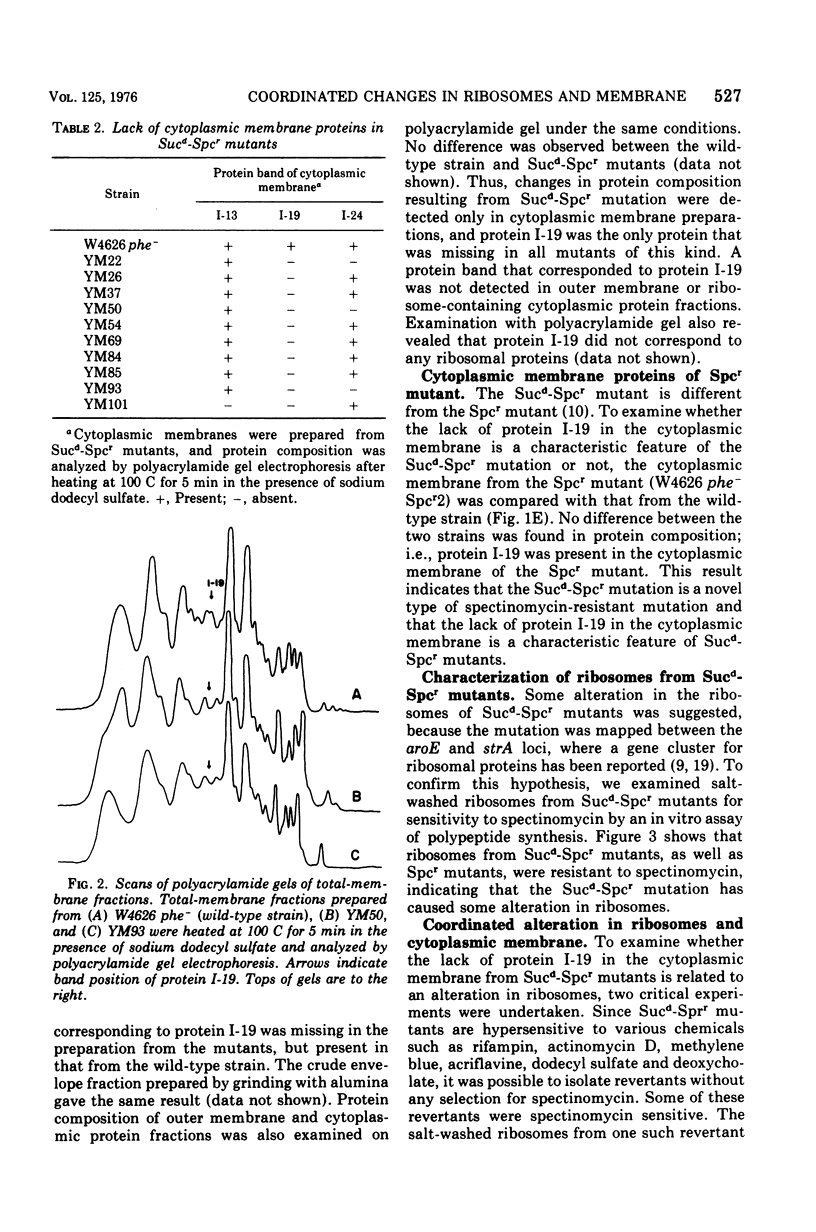
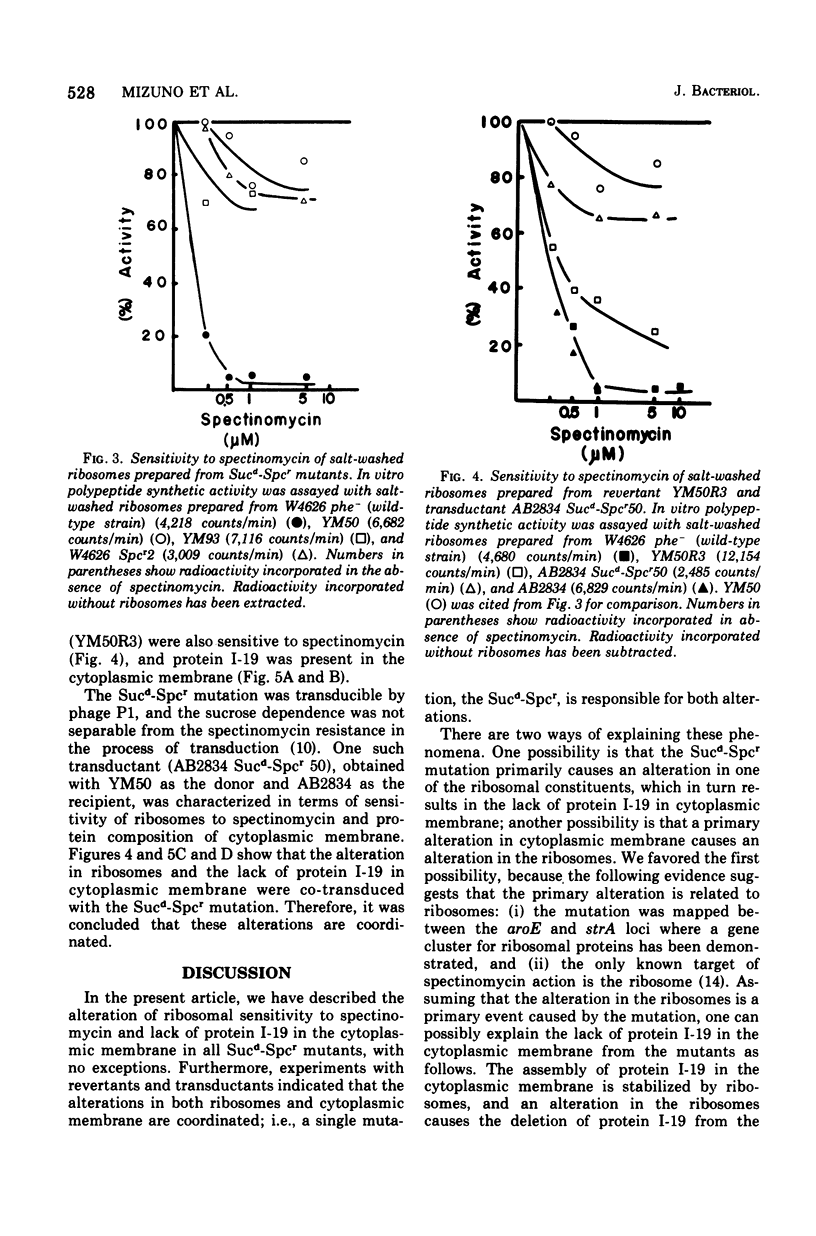
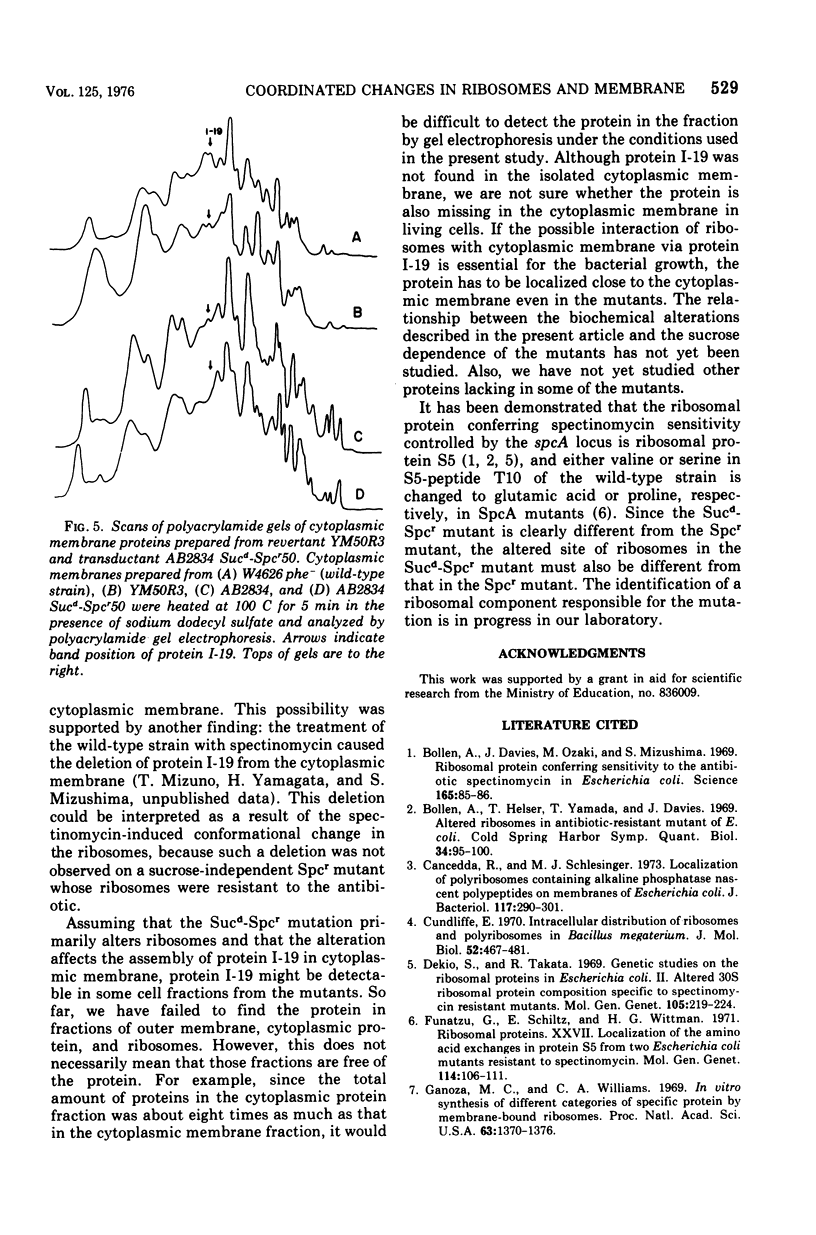
Alterations in cytoplasmic membrane and ribosomes from sucrose-dependent spectinomycin-resistant (Sucd-Spcr) mutants of Escherichia coli, mutants that are resistant to spectinomycin in the presence of 20% sucrose but sensitive in the absence of sucrose, were studied. The protein composition of cytoplasmic membrane was analyzed by gel electrophoresis on polyacrylamide gel containing 8 M urea and 0.5% sodium dodecyl sulfate, which assured the reproducible separation of 28 protein bands. A major protein band, I-19, was missing in all cytoplasmic membrane preparations from 10 Sucd-Spcr mutants. Besides protein I-19, proteins I-13 and I-24 were missing in some mutants. On the other hand, the protein composition of cytoplasmic membrane from a sucrose-independent spectinomycin-resistant mutant was indistinguishable from that from the wild-type strain. The polypeptide synthetic activity of ribosomes from Sucd-Spcr mutants was resistant to spectinomycin. Studies on a revertant obtained from one of these mutants without any selection for sensitivity to spectinomycin revealed that a single mutation was responsible for both the ribosomal alteration, i.e., spectinomycin resistance, and the lack of protein I-19 in the cytoplasmic membrane. Studies on a transductant obtained with a Sucd-SPcr mutant as the donor also confirmed the single-mutation concept. It was concluded that in Sucd-SPcr mutants an alteration in the ribosomes caused the deletion of protein I-19 from cytoplasmic membrane.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bollen A., Davies J., Ozaki M., Mizushima S. Ribosomal protein conferring sensitivity to the antibiotic spectinomycin in Escherichia coli. Science. 1968 Jul 4;165(3888):85–86. [PubMed] [Google Scholar]
- Bollen A., Helser T., Yamada T., Davies J. Altered ribosomes in antibiotic-resistant mutants of E. coli. Cold Spring Harb Symp Quant Biol. 1969;34:95–100. doi: 10.1101/sqb.1969.034.01.015. [DOI] [PubMed] [Google Scholar]
- Cancedda R., Schlesinger M. J. Localization of polyribosomes containing alkaline phosphatase nascent polypeptides on membranes of Escherichia coli. J Bacteriol. 1974 Jan;117(1):290–301. doi: 10.1128/jb.117.1.290-301.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cundliffe E. Intracellular distribution of ribosomes and polyribosomes in Bacillus megaterium. J Mol Biol. 1970 Sep 28;52(3):467–481. doi: 10.1016/0022-2836(70)90413-4. [DOI] [PubMed] [Google Scholar]
- Dekio S., Takata R. Genetic studies of the ribosomal proteins in Escherichia. coli. II. Altered 30s ribosomal protein component specific to spectinomycin resistant mutants. Mol Gen Genet. 1969;105(3):219–224. doi: 10.1007/BF00337473. [DOI] [PubMed] [Google Scholar]
- Funatsu G., Schiltz E., Wittmann H. G. Ribosomal proteins. XXVII. Localization of the amino acid exchanges in protein S5 from two Escherichia coli mutants resistant to spectinomycin. Mol Gen Genet. 1972;114(2):106–111. doi: 10.1007/BF00332781. [DOI] [PubMed] [Google Scholar]
- Ganoza M. C., Williams C. A. In vitro synthesis of different categories of specific protein by membrane-bound and free ribosomes. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1370–1376. doi: 10.1073/pnas.63.4.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki K., Sabol S., Wahba A. J., Ochoa S. Translation of the genetic message. VII. Role of initiation factors in formation of the chain initiation complex with Escherichia coli ribosomes. Arch Biochem Biophys. 1968 May;125(2):542–547. doi: 10.1016/0003-9861(68)90612-7. [DOI] [PubMed] [Google Scholar]
- Jaskunas S. R., Lindahl L., Nomura M. Specialized transducing phages for ribosomal protein genes of Escherichia coli. Proc Natl Acad Sci U S A. 1975 Jan;72(1):6–10. doi: 10.1073/pnas.72.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi Y., Yamagata H. Sucrose-dependent spectinomycin-resistant mutants of Escherichia coli. J Bacteriol. 1976 Jan;125(1):142–148. doi: 10.1128/jb.125.1.142-148.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T., Mizushima S. Characterization of stuck ribosomes induced by neomycin in vivo and in vitro. Biochim Biophys Acta. 1974 Jun 14;353(1):69–76. doi: 10.1016/0005-2787(74)90098-7. [DOI] [PubMed] [Google Scholar]
- Mizushima S., Yamada H. Isolation and characterization of two outer membrane preparations from Escherichia coli. Biochim Biophys Acta. 1975 Jan 14;375(1):44–53. doi: 10.1016/0005-2736(75)90071-1. [DOI] [PubMed] [Google Scholar]
- Nashimoto H., Nomura M. Structure and function of bacterial ribosomes. XI. Dependence of 50S ribosomal assembly on simultaneous assembly of 30S subunits. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1440–1447. doi: 10.1073/pnas.67.3.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka S. Inhibitors of ribosome functions. Annu Rev Microbiol. 1971;25:487–562. doi: 10.1146/annurev.mi.25.100171.002415. [DOI] [PubMed] [Google Scholar]
- Pittard J., Wallace B. J. Distribution and function of genes concerned with aromatic biosynthesis in Escherichia coli. J Bacteriol. 1966 Apr;91(4):1494–1508. doi: 10.1128/jb.91.4.1494-1508.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman C. M., Cherian M. G. The secretory pathways of rat serum glycoproteins and albumin. Localization of newly formed proteins within the endoplasmic reticulum. J Cell Biol. 1972 Feb;52(2):231–245. doi: 10.1083/jcb.52.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman C. M., Siekevitz P., Palade G. E. Synthesis and transfer of amylase in pigeon pancreatic micromosomes. J Biol Chem. 1966 Mar 10;241(5):1150–1158. [PubMed] [Google Scholar]
- SCHLESSINGER D., MARCHESTI V. T., KWAN B. C. BINDING OF RIBOSOMES TO CYTOPLASMIC RETICULUM OF BACILLUS MEGATERIUM. J Bacteriol. 1965 Aug;90:456–466. doi: 10.1128/jb.90.2.456-466.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varricchio F. "Compartmentalization" of Escherichia coli ribosomes and ribonucleic acid. J Bacteriol. 1972 Mar;109(3):1284–1294. doi: 10.1128/jb.109.3.1284-1294.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace B. J., Tai P. C., Davis B. D. Selective inhibition of initiating ribosomes by spectinomycin. Proc Natl Acad Sci U S A. 1974 May;71(5):1634–1638. doi: 10.1073/pnas.71.5.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata H., Uchida H. Chromosomal mutations affecting the stability of sex-factors in Escherichia coli. J Mol Biol. 1972 Jan 28;63(2):281–294. doi: 10.1016/0022-2836(72)90375-0. [DOI] [PubMed] [Google Scholar]
- Yamagata H., Uchida H. Spectinomycin resistance mutations affecting the stability of sex-factors in Escherichia coli. J Mol Biol. 1972 Jun 28;67(3):533–535. doi: 10.1016/0022-2836(72)90472-x. [DOI] [PubMed] [Google Scholar]
- van Knippenberg P. H., Duijts G. A., Euwe M. S. Polyribosomes of Escherichia coli. I. Isolation of polysomes from a complex of DNA and membrane. Mol Gen Genet. 1971;112(3):197–207. doi: 10.1007/BF00269172. [DOI] [PubMed] [Google Scholar]


