Abstract
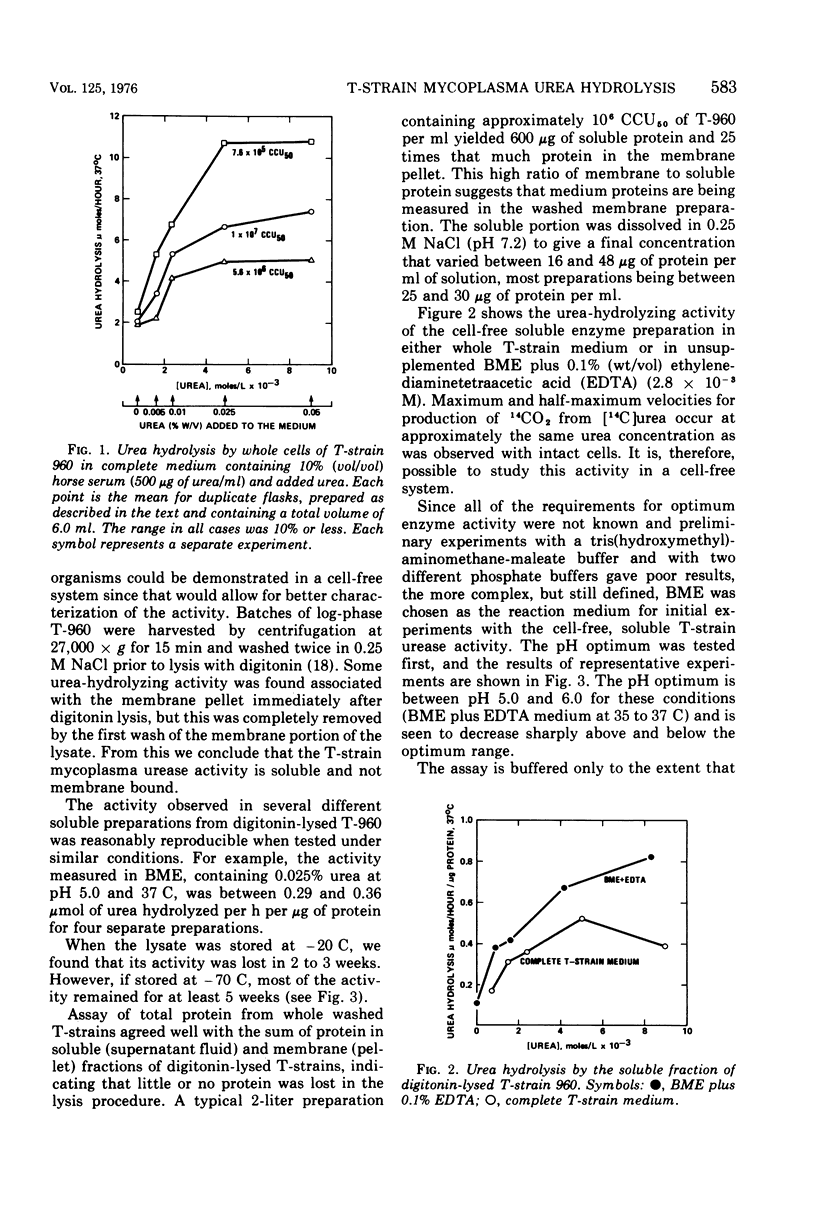
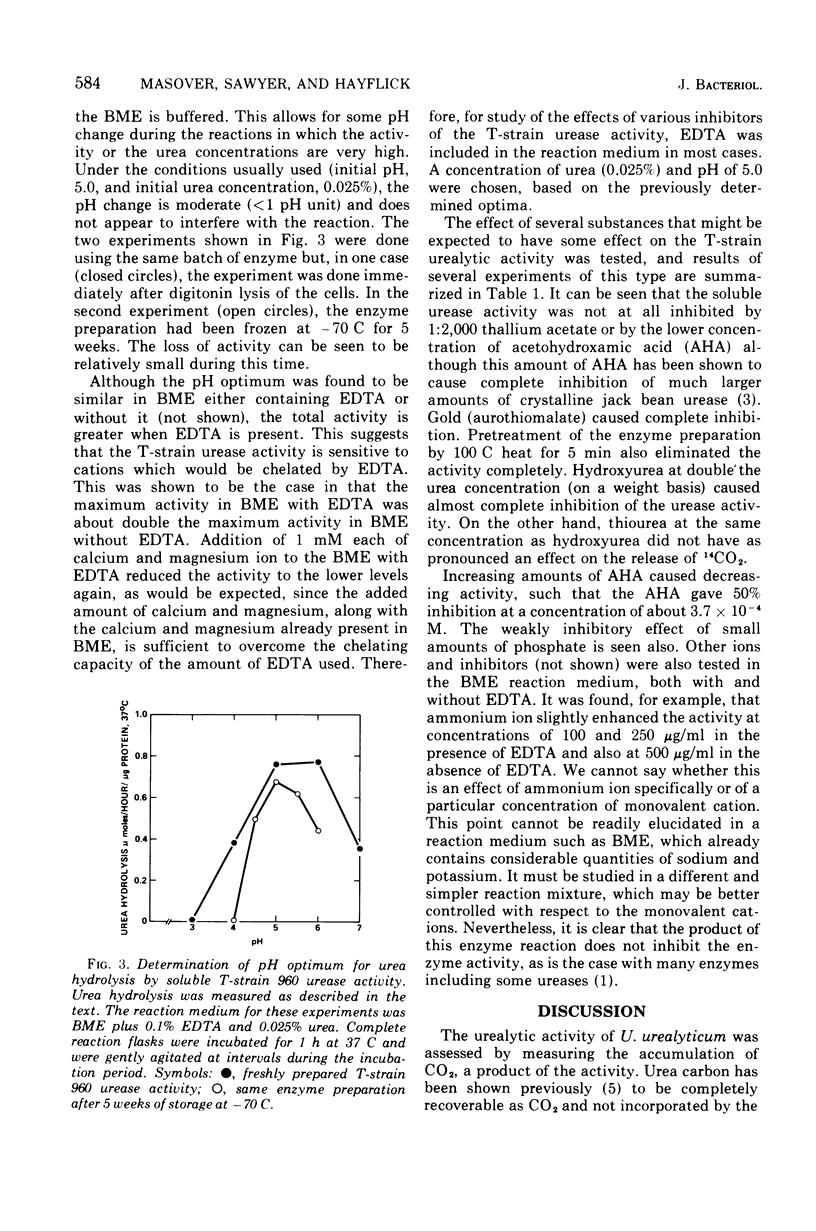
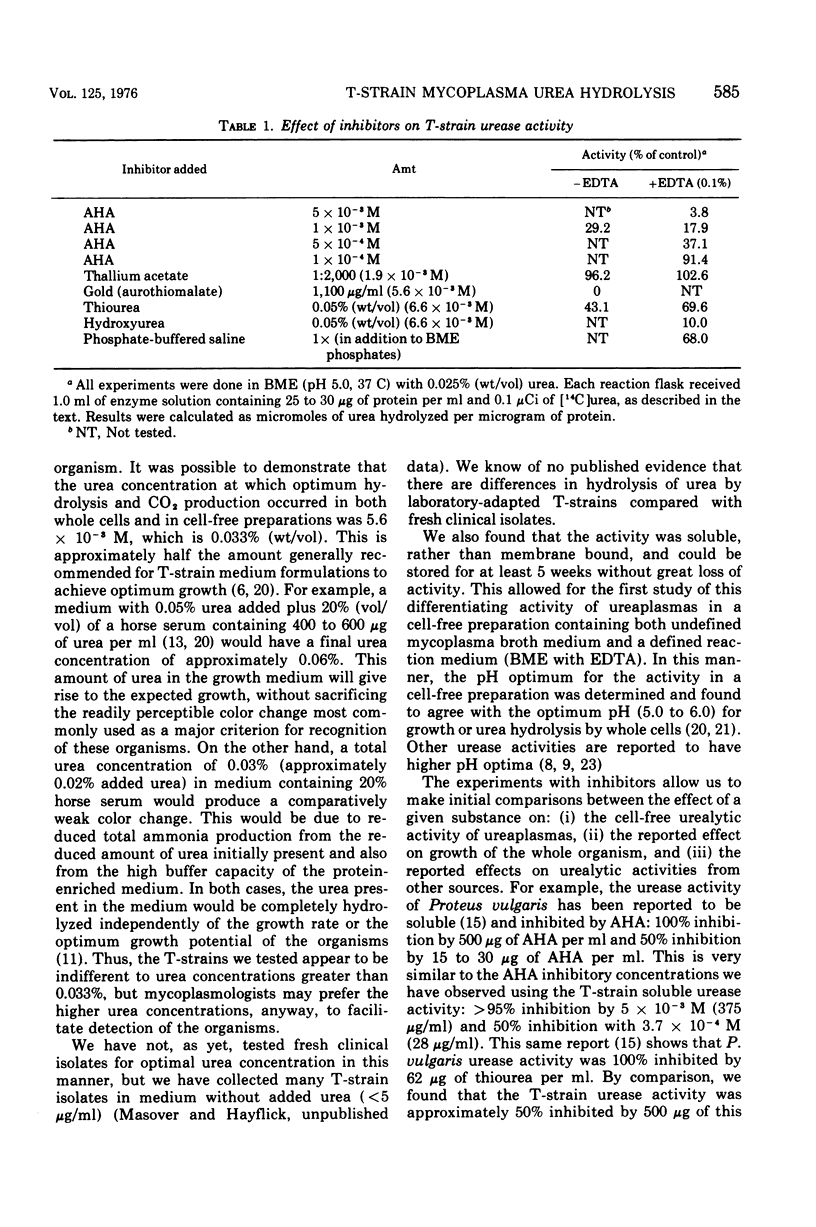
The urea-hydrolyzing activity of a T-strain mycoplasma was studied in experiments using whole cells and cell-free enzyme preparations by measuring the release of 14CO2 from [14C]urea. Under the conditions used, the urea concentration optimum is approximately 5.6 X 10(-3) M urea. The activity is soluble and not membrane bound. It is stable at -70 C for several weeks but is more labile at higher temperatures. The pH optimum is between 5.0 and 6.0. The effect of several inhibitors on the activity was tested and revealed similarities, as well as differences, between T-strain mycoplasma urease activity and the urease activity of other organisms and plants.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chin C. C., Gorin G. Urease. VII. Some observations on the assay method of Sumner. Anal Biochem. 1966 Oct;17(1):60–65. doi: 10.1016/0003-2697(66)90007-8. [DOI] [PubMed] [Google Scholar]
- FISHBEIN W. N., CARBONE P. P. UREASE CATALYSIS. II. INHIBITION OF THE ENZYME BY HYDROXYUREA, HYDROXYLAMINE, AND ACETOHYDROXAMIC ACID. J Biol Chem. 1965 Jun;240:2407–2414. [PubMed] [Google Scholar]
- FISHBEIN W. N., WINTER T. S., DAVIDSON J. D. UREASE CATALYSIS. I. STOICHIOMETRY, SPECIFICITY, AND KINETICS OF A SECOND SUBSTRATE: HYDROXYUREA. J Biol Chem. 1965 Jun;240:2402–2406. [PubMed] [Google Scholar]
- Fishbein W. N. Urease catalysis. 3. Stoichiometry, kinetics, and inhibitory properties of a third substrate: dihydroxyurea. J Biol Chem. 1969 Mar 10;244(5):1188–1193. [PubMed] [Google Scholar]
- Ford D. K., MacDonald J. Influence of urea on the growth of T-strain mycoplasmas. J Bacteriol. 1967 May;93(5):1509–1512. doi: 10.1128/jb.93.5.1509-1512.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford D. K., McCandlish K. L., Gronlund A. F. Metabolism of 14C-urea by T-strain mycoplasma. J Bacteriol. 1970 May;102(2):605–606. doi: 10.1128/jb.102.2.605-606.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayflick L. Tissue cultures and mycoplasmas. Tex Rep Biol Med. 1965 Jun;23(Suppl):285+–285+. [PubMed] [Google Scholar]
- LARSON A. D., KALLIO R. E. Purification and properties of bacterial urease. J Bacteriol. 1954 Jul;68(1):67–73. doi: 10.1128/jb.68.1.67-73.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LISTER A. J. The kinetics of urease activity in Corynebacterium renale. J Gen Microbiol. 1956 Apr;14(2):478–484. doi: 10.1099/00221287-14-2-478. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Masover G. K., Benson J. R., Hayflick L. Growth of T-strain mycoplasmas in medium without added urea: effect of trace amounts of urea and of a urease inhibitor. J Bacteriol. 1974 Feb;117(2):765–774. doi: 10.1128/jb.117.2.765-774.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masover G. K., Hayflick L. Dialysis culture of T-strain mycoplasmas. J Bacteriol. 1974 Apr;118(1):46–52. doi: 10.1128/jb.118.1.46-52.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masover G. K., Mischak R. P., Hayflick L. Some effects of growth medium composition on the antigenicity of a T-strain mycoplasma. Infect Immun. 1975 Mar;11(3):530–539. doi: 10.1128/iai.11.3.530-539.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pianotti R. S., Mohan R. R., Schwartz B. S. Proteus vulgaris urease: in vitro inhibition by urea analogues. Proc Soc Exp Biol Med. 1966 Jun;122(2):506–508. doi: 10.3181/00379727-122-31174. [DOI] [PubMed] [Google Scholar]
- Purcell R. H., Taylor-Robinson D., Wong D., Chanock R. M. Color test for the measurement of antibody to T-strain mycoplasmas. J Bacteriol. 1966 Jul;92(1):6–12. doi: 10.1128/jb.92.1.6-12.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roon R. J., Levenberg B. CO2 fixation and the involvement of allophanate in the biotin-enzyme-catalyzed cleavage of urea. J Biol Chem. 1970 Sep 10;245(17):4593–4595. [PubMed] [Google Scholar]
- Rottem S., Razin S. Isolation of mycoplasma membranes by digitonin. J Bacteriol. 1972 May;110(2):699–705. doi: 10.1128/jb.110.2.699-705.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard M. C. Human mycoplasma infections. Health Lab Sci. 1966 Jul;3(3):163–169. [PubMed] [Google Scholar]
- Shepard M. C., Lunceford C. D. Occurrence of urease in T strains of Mycoplasma. J Bacteriol. 1967 May;93(5):1513–1520. doi: 10.1128/jb.93.5.1513-1520.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]


