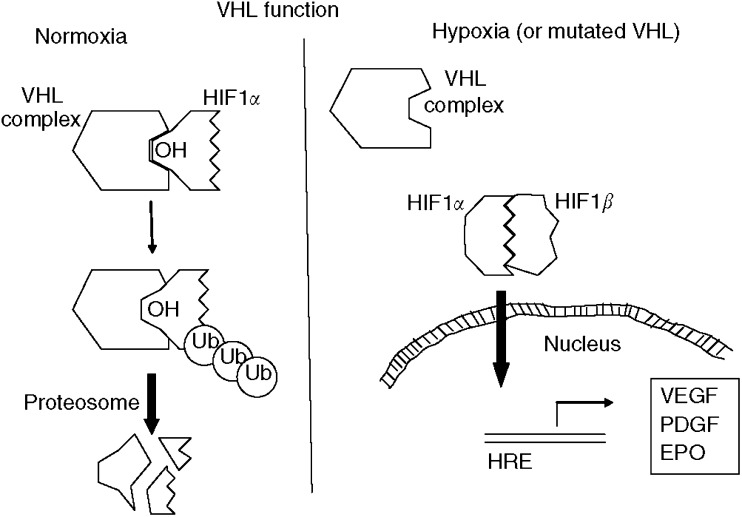
Figure 1.
VHL and HIF-1 pathways. The VHL complex (composed of von Hippel–Lindau protein, elongin B, elongin C, Cul2, and Rbx1) functions to regulate levels of hypoxia-inducible factor (HIF)-1α. During normoxia, HIF-1α is hydroxylated at two proline residues via an oxygen-dependent enzymatic mechanism. The VHF complex binds to the hydroxylated HIF-1α and polyubiquinates HIF-1α, leading to proteosome-mediated degradation of HIF-1α. During hypoxia, HIF-1α is not hydroxylated, and thus cannot bind with the VHL complex. HIF-1α accumulates and binds to HIF-1β, thus forming the HIF-1 complex, which subsequently translocates into the cell nucleus where it binds with hypoxia-responsive element (HRE) in gene promotors and facilitates expression of hypoxia-inducible genes. Similarly, loss of function mutations of VHL prevents ubiquitin-mediated degradation of HIF-1α, resulting in upregulation of hypoxia-inducible genes.