Abstract
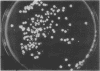
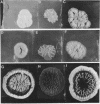
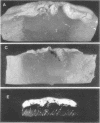
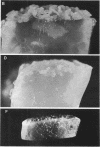
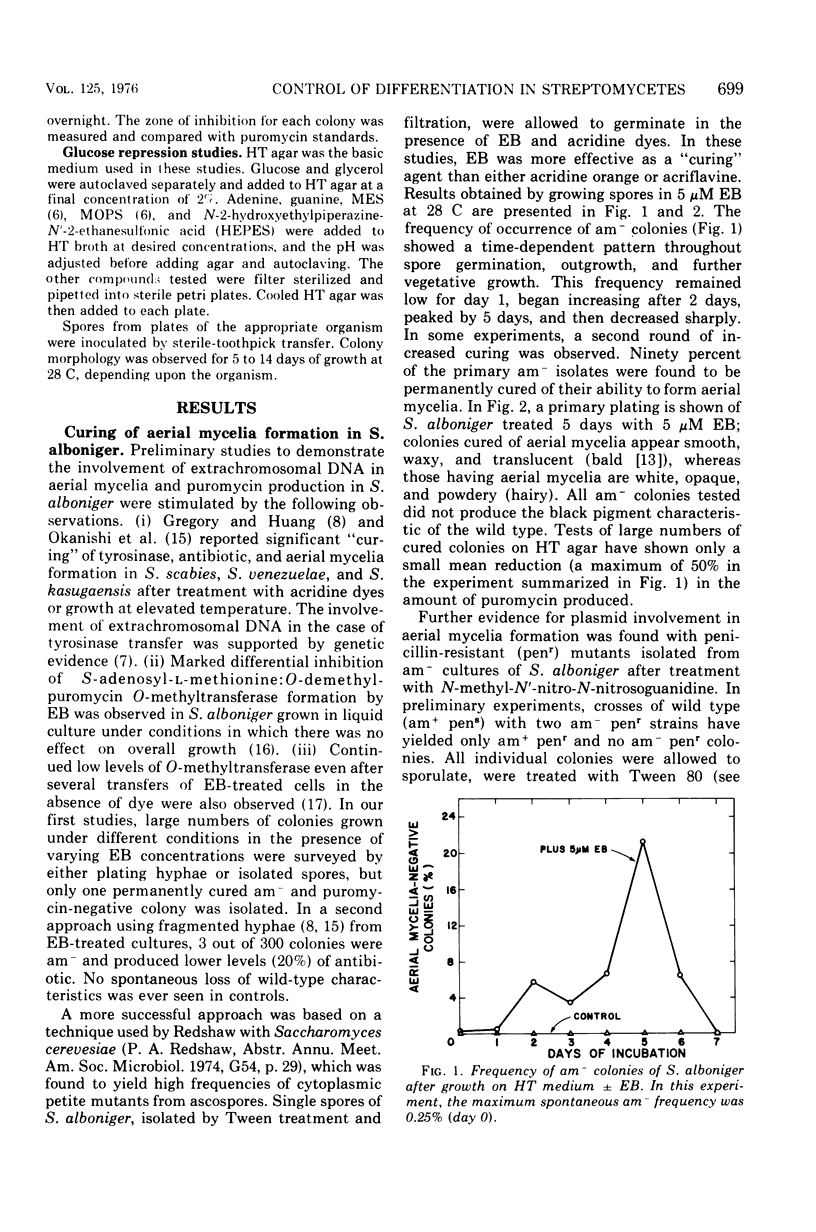
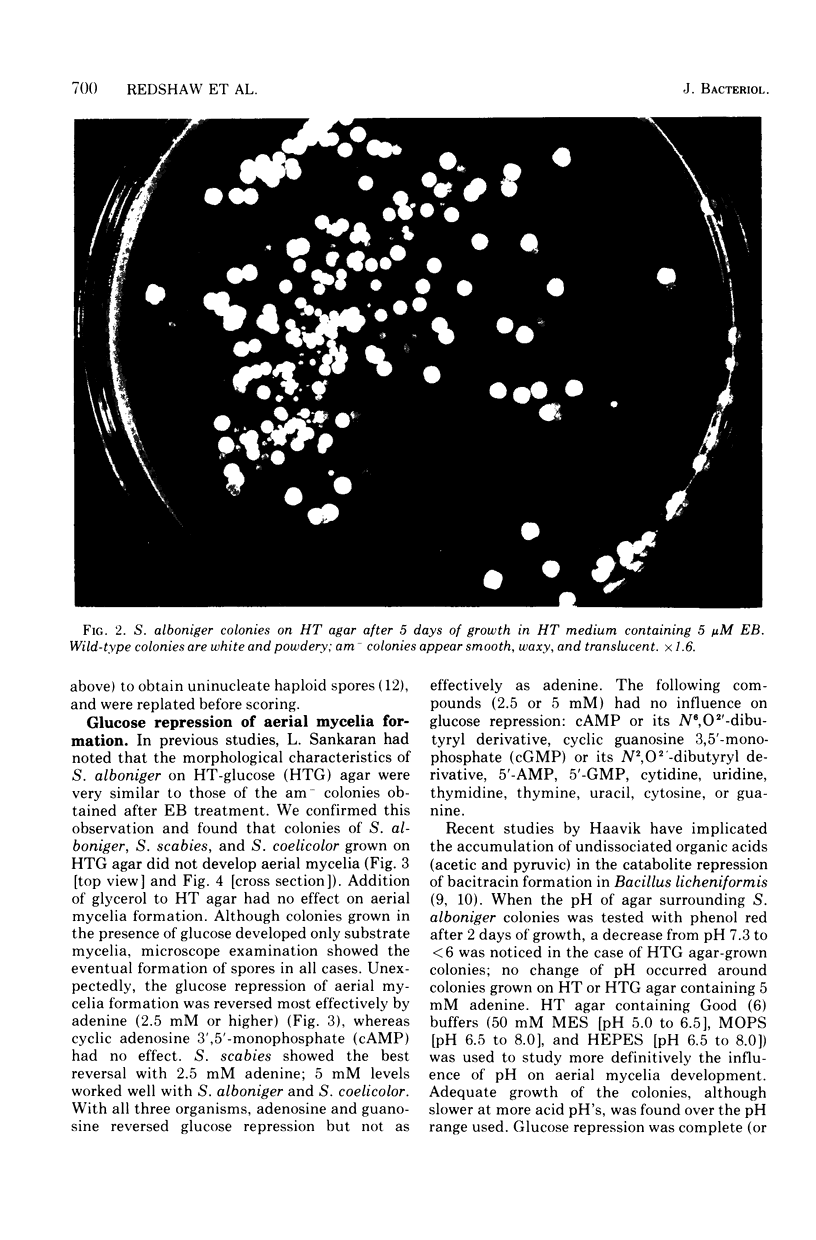
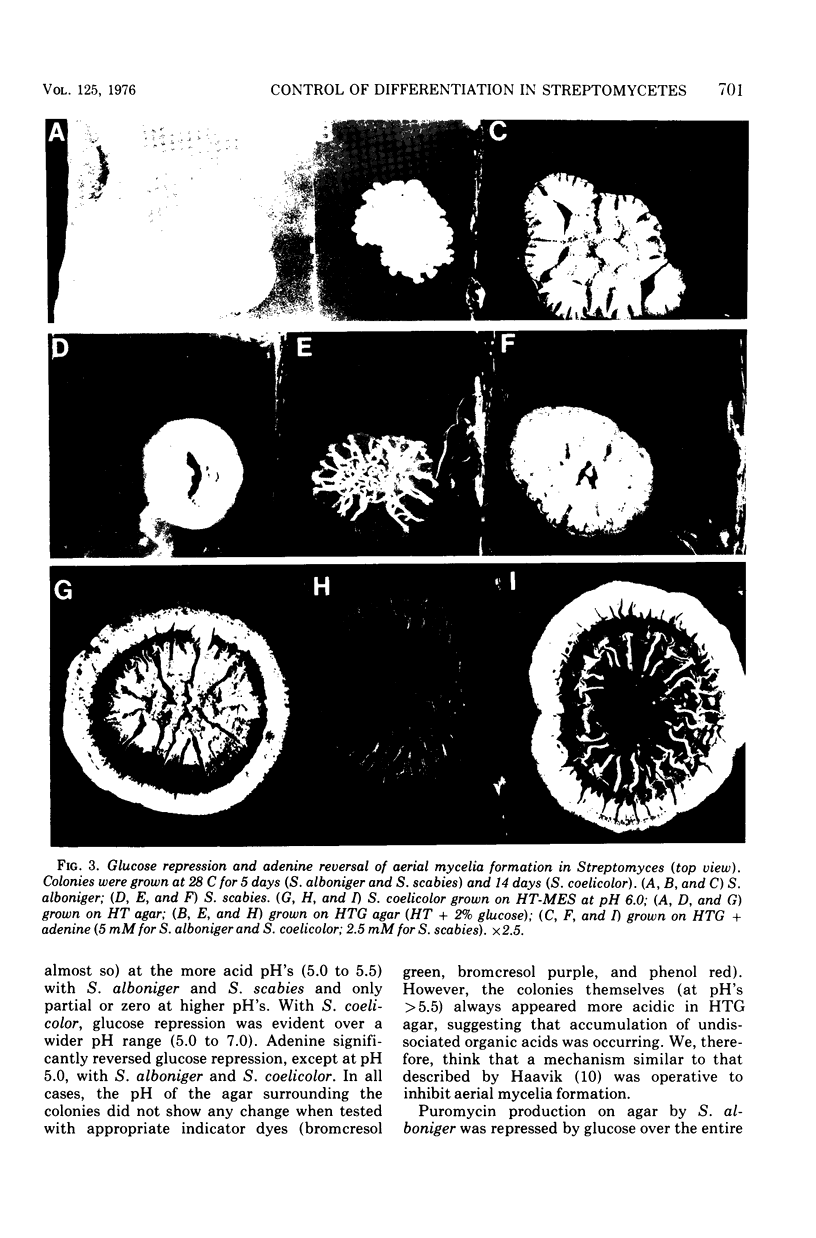
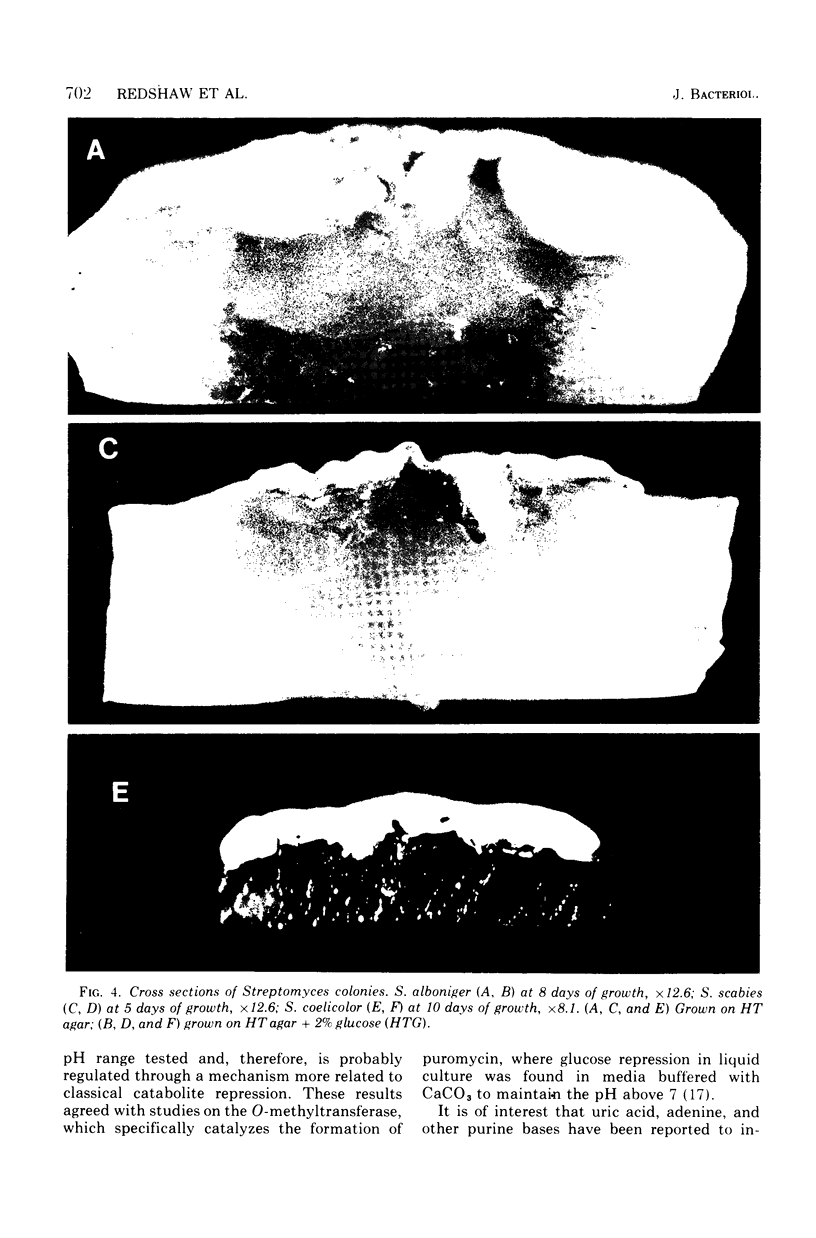
When Streptomyces alboniger spores were grown in Hickey-Tresner broth containing 5 muM ethidium bromide, a high frequency of permanently cured aerial mycelia-negative (am-) colonies was recovered. The appearance an am- colonies was time dependent: a very low frequency (0.3%) at zero time, a maximum (9 to 21%) after 2 to 5 days of growth, and a decline again to low frequencies later in the growth cycle. On agar, cured am- colonies of S. alboniger still produced puromycin. The development of aerial mycelia in S. alboniger, S. scabies, and S. coelicolor was also sensitive to glucose repression. Colonies grown on Hickey-Tresner agar containing 2% glucose remained phenotypically am- throughout the observation period. Adenine (2.5 mM or greater), and to a lesser extent adenosine and guanosine, specifically reversed the repression. The accumulation of undissociated organic acids appears to be involved in glucose repression of aerial mycelia formation. However, this does not appear to be the case with puromycin production in S. alboniger; glucose repression was observed over the pH range 5.0 to 7.5.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernlohr R. W., Haddox M. K., Goldberg N. D. Cyclic guanosine 3':5'-monophosphate in Escherichia coli and Bacillus lichenformis. J Biol Chem. 1974 Jul 10;249(13):4329–4331. [PubMed] [Google Scholar]
- Campos J. M., Zusman D. R. Regulation of development in Myxococcus xanthus: effect of 3':5'-cyclic AMP, ADP, and nutrition. Proc Natl Acad Sci U S A. 1975 Feb;72(2):518–522. doi: 10.1073/pnas.72.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. B., Gross R., Su Y. F., Perkins J. P. Regulation of adenosine 3':5'-monophosphate content in human astrocytoma cells by adenosine and the adenine nucleotides. J Biol Chem. 1974 Aug 25;249(16):5296–5303. [PubMed] [Google Scholar]
- Durieu-Trautmann O., Tavlitzki J. Reversible and permanent effects of the carbon sources and various antibiotics on the morphology and metabolic properties of Ustilago cynodontis cells. J Cell Biol. 1975 Jul;66(1):102–113. doi: 10.1083/jcb.66.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman J. F., Thayer J. P. Cyclic AMP-induced tyrosinase synthesis in Neurospora crassa. Biochem Biophys Res Commun. 1974 Dec 11;61(3):977–982. doi: 10.1016/0006-291x(74)90251-4. [DOI] [PubMed] [Google Scholar]
- GREGORY K. F., HUANG J. C. TYROSINASE INHERITANCE IN STREPTOMYCES SCABIES. I. GENETIC RECOMBINATION. J Bacteriol. 1964 Jun;87:1281–1286. doi: 10.1128/jb.87.6.1281-1286.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREGORY K. F., HUANG J. C. TYROSINASE INHERITANCE IN STREPTOMYCES SCABIES. II. INDUCTION OF TYROSINASE DEFICIENCY BY ACRIDINE DYES. J Bacteriol. 1964 Jun;87:1287–1294. doi: 10.1128/jb.87.6.1287-1294.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerisch G., Fromm H., Huesgen A., Wick U. Control of cell-contact sites by cyclic AMP pulses in differentiating Dictyostelium cells. Nature. 1975 Jun 12;255(5509):547–549. doi: 10.1038/255547a0. [DOI] [PubMed] [Google Scholar]
- Good N. E., Winget G. D., Winter W., Connolly T. N., Izawa S., Singh R. M. Hydrogen ion buffers for biological research. Biochemistry. 1966 Feb;5(2):467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- HICKEY R. J., TRESNER H. D. A cobalt-containing medium for sporulation of Streptomyces species. J Bacteriol. 1952 Dec;64(6):891–892. doi: 10.1128/jb.64.6.891-892.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haavik H. I. Studies on the formation of bacitracin by Bacillus licheniformis: effect of glucose. J Gen Microbiol. 1974 Apr;81(2):383–390. doi: 10.1099/00221287-81-2-383. [DOI] [PubMed] [Google Scholar]
- Haavik H. I. Studies on the formation of bacitracin by Bacillus licheniformis: role of catabolite repression and organic acids. J Gen Microbiol. 1974 Oct;84(2):321–326. doi: 10.1099/00221287-84-2-321. [DOI] [PubMed] [Google Scholar]
- Hopwood D. A., Chater K. F., Dowding J. E., Vivian A. Advances in Streptomyces coelicolor genetics. Bacteriol Rev. 1973 Sep;37(3):371–405. doi: 10.1128/br.37.3.371-405.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood D. A. Genetic analysis and genome structure in Streptomyces coelicolor. Bacteriol Rev. 1967 Dec;31(4):373–403. doi: 10.1128/br.31.4.373-403.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kähler R., Noack D. Action of acridine orange and ethidium bromide on growth and antibiotic activity of Streptomyces hygroscopicus JA 6599. Z Allg Mikrobiol. 1974;14(6):529–533. doi: 10.1002/jobm.3630140610. [DOI] [PubMed] [Google Scholar]
- Okanishi M., Ita T., Umezawa H. Possible control of formation of aerial mycelium and antibiotic production in Streptomyces by episomic factors. J Antibiot (Tokyo) 1970 Jan;23(1):45–47. doi: 10.7164/antibiotics.23.45. [DOI] [PubMed] [Google Scholar]
- Sankaran L., Pogell B. M. Biosynthesis of puromycin in Streptomyces alboniger: regulation and properties of O-demethylpuromycin O-methyltransferase. Antimicrob Agents Chemother. 1975 Dec;8(6):721–732. doi: 10.1128/aac.8.6.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaran L., Pogell B. M. Differential inhibition of catabolite-sensitive enzyme induction by intercalating dyes. Nat New Biol. 1973 Oct 31;245(148):257–260. doi: 10.1038/newbio245257a0. [DOI] [PubMed] [Google Scholar]
- Schrempf H., Bujard H., Hopwood D. A., Goebel W. Isolation of covalently closed circular deoxyribonucleic acid from Streptomyces coelicolor A3(2). J Bacteriol. 1975 Feb;121(2):416–421. doi: 10.1128/jb.121.2.416-421.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro L., Agabian-Keshishian N., Hirsch A., Rosen O. M. Effect of dibutyryladenosine 3':5'-cyclic monophosphate on growth and differentiation in Caulobacter crescentus. Proc Natl Acad Sci U S A. 1972 May;69(5):1225–1229. doi: 10.1073/pnas.69.5.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terenzi H. F., Flawiá M. M., Torres H. N. A Neurospora crassa morphological mutant showing reduced adenylate cyclase activity. Biochem Biophys Res Commun. 1974 Jun 18;58(4):990–996. doi: 10.1016/s0006-291x(74)80241-x. [DOI] [PubMed] [Google Scholar]