FIGURE 5. The Sbh1p-TM domain is sufficient for co-precipitation with Sec61 translocation complex but not for functional interaction the exocyst.
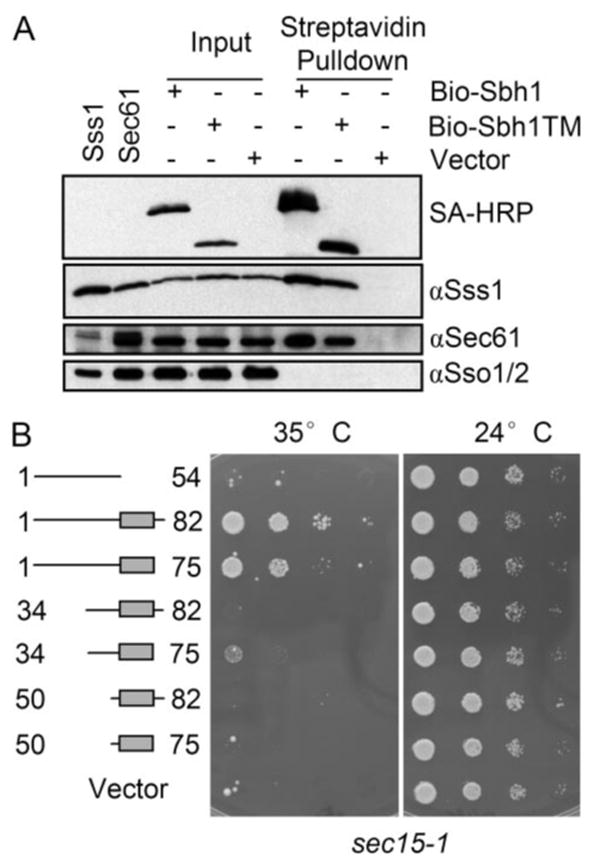
A, digitonin-solubilized membranes of SBH1 deleted cells (H3227) expressing N-terminal BIO-tagged full-length Sbh1p (amino acids 1–82), TM domain (amino acids 50–75), or the empty vector were subjected to pull-down with streptavidin-coated magnetic beads. Beads and input samples were resolved by SDS-PAGE, transferred to nitrocellulose, and analyzed by Western blotting with antibodies to Sec61p, Sss1p, or Sso1/2p, or with HRP-conjugated streptavidin to detect BIO-Sbh1p. The first two lanes are whole cell lysates overexpressing either Sss1p or Sec61p. B, the sec15–1 cells carrying YEpSBH1-(1–54), YEpSBH1-(34–75), YEpSBH1-(34–82), YEpSBH1-(50–75), YEpSBH1-(50–82), YEpSBH1-(1–82) or the empty vector pVT102U were grown on SCD-Ura plates at different temperatures. Sbh1p-TM or the cytosolic domain alone cannot suppress the temperature-sensitive growth phenotype of sec15-1 cells in contrast to full-length Sbh1p and the sbh1 mutant lacking the luminal part.
