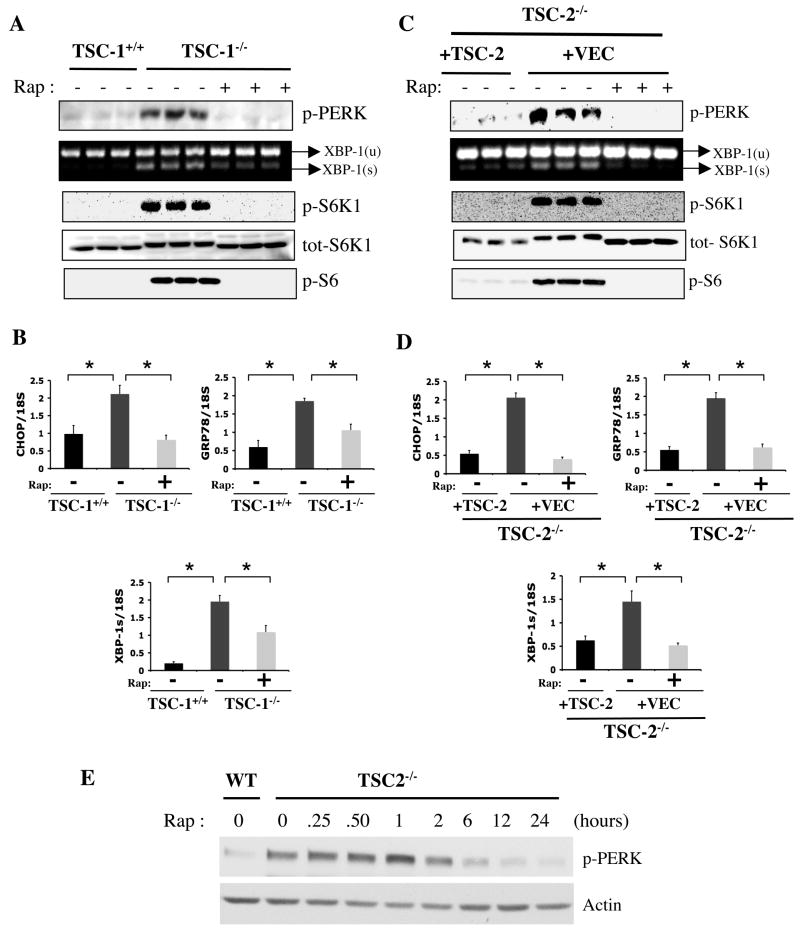
Figure 1. Analysis of UPR and mTORC1 signaling pathways in Tsc1−/− and Tsc2−/− cells.
(A) PERK (Thr980) phosphorylation, XBP-1 mRNA splicing and downstream elements of mTORC1 signaling; phosphorylation of S6K1 (Thr389), total S6K1 levels and S6 (Ser235/236) phosphorylation in the presence or absence of rapamycin (20 nM) for 24 hours in Tsc1−/− MEFs and WT controls (triplicates shown). (B) Expression levels of CHOP, GRP78, and XBP-1s mRNAs in Tsc1+/+ and Tsc1−/− cells treated either with rapamycin or vehicle. (C) UPR and mTOR signaling parameters in Tsc2−/− MEFs reconstituted with a retroviral control vector (+VEC) or with a retrovirus containing the WT TSC2 gene. (D) CHOP, GRP78, and XBP-1s mRNA levels in TSC2-deficient cells reconstituted with either vector or WT TSC2 upon treatment with rapamycin (20 nM) or vehicle, as above. (E) Time course of rapamycin’s effect on PERK phosphorylation in Tsc2−/− MEFs. Tsc2+/+ (WT) or Tsc2−/− cells were serum starved for 24 h in the presence of rapamycin (20 nM) for the duration indicated. Data are mean ± SEM of triplicates.