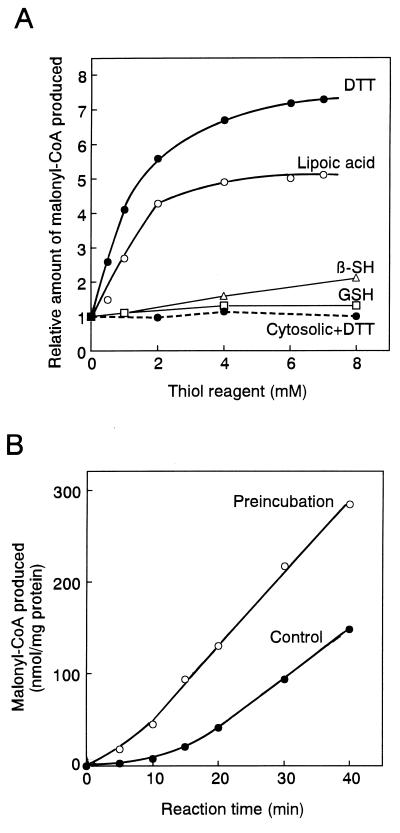
Figure 1.
Effects of thiol reagents on the ACCase activity. (A) ACCase activity was measured in the indicated concentration of a thiol reagent as described in Materials and Methods. Plastidic ACCase from pea, continuous lines; cytosolic ACCase from pea, dashed line. β-SH, 2-mercaptoethanol; GSH, reduced form of glutathione. Lipoic acid was dissolved into 50 mM Tricine⋅KOH (pH 8) containing 1 mM phenylmethylsulfonyl fluoride, 1 mM benzamide, and 5 mM ɛ-amino-n-caproic acid at 20 mM, and 0–7 μl of 20 mM solution was added to 13-μl reaction mixture. The activity without any thiol reagent was 11 nmol/20 min per mg of protein for pea plastidic ACCase and 210 nmol/20 min per mg of protein for pea cytosolic ACCase. The activity is relative to that without a thiol reagent, taken to be 1. Results are the means of duplicate determinations that differed less than 10%. (B) The effects of preincubation of plastidic ACCase with DTT on activity were examined. Changes with time are shown. After the enzyme was incubated at 30°C for 10 min with 2 mM DTT, the reaction was started by the addition of buffer, ATP, MgCl2, KCl, acetyl-CoA, and NaH14CO3. The final concentrations of these reagents were the same as above. The final concentration of DTT was 1 mM.