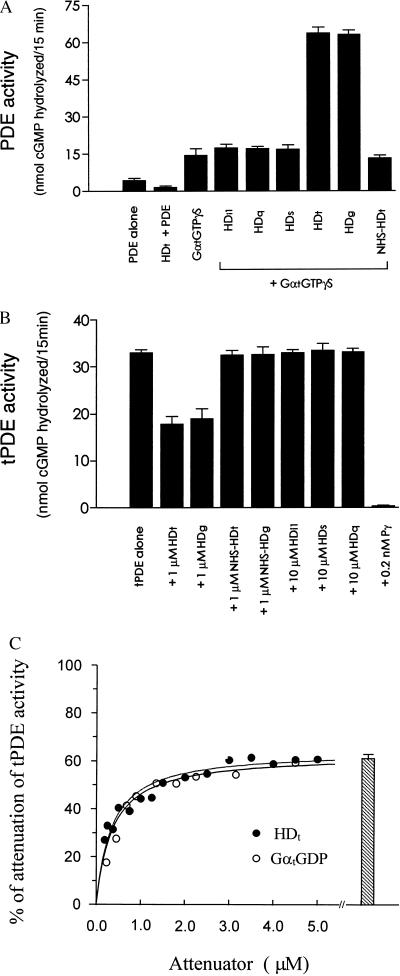
Figure 2.
Selective HD regulation of PDE. (A) Selectivity of HD synergy with GαtGTPγS. The enzymatic activity of PDE (0.5 nM) was determined with the indicated additions to the reactions. The concentrations for HDi1, HDq, HDs, and NHS-HDt were 2.0 μM, HDt and HDg were 1.0 μM, and GαtGTPγS was 0.45 μM. Error bars indicate ±SEM derived from values obtained in three independent experiments, with each determination performed in triplicate. PDE activity assay was performed as described in Materials and Methods. The GαtGTPγS was prepared from the purified GαtGDP as described (47). (B) Selective interaction of the HDs with PDE catalytic core. Reactivity of the HD proteins isolated from different Gα families toward PDE catalytic core was determined with 0.2 nM tPDE in the presence of the indicated HD concentrations. Limited trypsinization to produce tPDE was performed as described in Materials and Methods. Each value is the mean ± SEM of data obtained from three independent experiments. (C) Attenuation of the catalytic core enzyme by HDt and GαtGDP. Attenuation of 0.2 nM tPDE was determined in the presence of the indicated concentrations of either HDt (•) or GαtGDP (○). The activity for each condition is expressed as the fractional inhibition of the activity assayed for the tPDE alone (34.2 ± 1 nmol of cGMP hydrolyzed). The bar presents the fractional inhibition obtained in the presence of 4.5 μM GαtGDP and 5 μM HDt. The error bars are ±SEM for values obtained in three independent experiments. The curves are representative of the saturations from three independent experiments.