Abstract
To explore the role of the Rho GTPases in lens morphogenesis, we overexpressed bovine Rho GDP dissociation inhibitor (RhoGDIα), which serves as a negative regulator of Rho, Rac and Cdc42 GTPase activity, in a lens-specific manner in transgenic mice. This was achieved using a chimeric promoter of δ-crystallin enhancer and αA-crystallin, which is active at embryonic day 12. Several individual transgenic (Tg) lines were obtained, and exhibited ocular specific phenotype comprised of microphthalmic eyes with lens opacity. The overexpression of bovine RhoGDIα disrupted membrane translocation of Rho, Rac and Cdc42 GTPases in Tg lenses. Transgenic lenses also revealed abnormalities in the migration pattern, elongation and organization of lens fibers. These changes appeared to be associated with impaired organization of the actin cytoskeleton and cell-cell adhesions. At E14.5, the size of the RhoGDIα Tg lenses was larger compared to wild type (WT) and the central lens epithelium and differentiating fibers exhibited an abnormal increase of bromo-deoxy-uridine incorporation. Postnatal Tg eyes, however, were much smaller in size compared to WT eyes, revealing increased apoptosis in the disrupted lens fibers. Taken together, these data demonstrate a critical role for Rho GTPase dependent signaling pathways in processes underlying morphogenesis, fiber cell migration, elongation and survival in the developing lens.
Keywords: Rho GTPases, RhoGDIα, Lens, Cytoskeleton, Elongation, Differentiation, Migration and Transgenic Mouse
Introduction
Lens fiber cell morphology, migration, membrane remodeling and intercellular adhesions are some of the key determinants of lens shape, distinct architecture and ultimately, optical properties. During lens development, the newly formed lens epithelial cells at the germinative zone migrate from the equator, elongate and differentiate into fiber cells. Lens fiber cell elongation and differentiation is associated with a dramatic change in cell morphology, with the length of fiber cells increasing on the order of several hundred-fold (Chow and Lang, 2001; Lovicu and McAvoy, 2005; Piatigorsky, 1981). These morphological changes are associated with membrane cytoskeleton remodeling, actin filament assembly and cell adhesion turnover (Beebe et al., 2001; Lee et al., 2000; Mousa and Trevithick, 1977; Perng and Quinlan, 2005; Ramaekers et al., 1981; Rao and Maddala, 2006; Weber and Menko, 2006a). In addition to morphological changes, the tips of the elongating fiber cells at both anterior and posterior terminals slide along the epithelium and capsule, respectively, as these cells migrate inward, and finally detach at the suture where they form contacts with their counterparts from the opposite side of the lens (Bassnett et al., 1999; Lee et al., 2000). These cell movements are fundamental for maintaining distinct lens fiber cell polarity. Since lens grows throughout life, lens epithelial cell proliferation, migration and differentiation are continuous events that are temporally and spatially regulated (Chow and Lang, 2001; Lovicu and McAvoy, 2005; Piatigorsky, 1981). It is recognized that lens epithelial cells undergoing elongation and differentiation exhibit a distinct pattern of actin cytoskeletal reorganization (Courtois et al., 1981; Lee et al., 2000; Mousa and Trevithick, 1977; Ramaekers et al., 1982; Weber and Menko, 2006a), and disruption of actin cytoskeletal organization impairs lens epithelial elongation and differentiation, indicating the importance of actin filament organization for these processes (Mousa and Trevithick, 1977). Although important insights have emerged regarding external cues controlling lens epithelial cell proliferation, migration and differentiation, little is known about the signaling pathways regulating fiber cell migration, adhesive interactions, cytoskeletal reorganization, cell shape changes, and polarity (Bassnett et al., 1999; Beebe et al., 2001; Lee et al., 2000; Perng and Quinlan, 2005; Piatigorsky, 1981; Rao and Maddala, 2006; Sue Menko, 2002; Weber and Menko, 2006a; Zelenka, 2004).
Rho GTPases are GTP-binding proteins that control many features of cell behavior in all eukaryotic cells. They play a pivotal role in regulating actin cytoskeletal organization, cell adhesive interactions, and influence cell polarity, morphogenesis, migration, vesicle trafficking, cell cycle progression and transcriptional activity (Burridge and Wennerberg, 2004; Cernuda-Morollon and Ridley, 2006; Hall, 2005; Van Aelst and D’Souza-Schorey, 1997). The Rho GTPases (Rho, Rac and Cdc42), which have been extensively studied, have specific functions attributed to each of the three GTPases in various cell types. These include regulation of actin stress fiber and focal adhesion formation, and cell motility by Rho GTPase; regulation of membrane ruffling, lamellipodium formation, actin polymerization, and cadherin-mediated cell-cell adhesion by Rac GTPase, and control of filopodium formation, microtubule-dependent cell polarization and migration by Cdc42 (Burridge and Wennerberg, 2004; Etienne-Manneville and Hall, 2002). Based on this understanding, it is widely suggested that the Rho GTPases play a critical role in cellular processes that are dependent on actin cytoskeletal organization (Hall, 2005). Lens epithelial and fiber cells express the RhoA, RhoB, Rac1 and Cdc42 GTPases (Chen et al., 2006; Maddala et al., 2001) and the activities of these GTPases is known to be stimulated by various growth factors involved in the regulation of lens growth and differentiation (Maddala et al., 2003).
The Rho GTPases act as molecular switches by cycling between active GTP-bound and inactive GDP-bound forms. This cycling is regulated by guanine nucleotide exchange factors (GEFs) which serve as activators, and GTPase activating proteins (GAPs) and GDP dissociation inhibitors (GDIs), which play the role of negative regulators of Rho GTPase activity (Burridge and Wennerberg, 2004; Etienne-Manneville and Hall, 2002). In their GTP-bound form, the Rho GTPases interact with specific downstream effector proteins, which include protein kinases and regulators of actin polymerization (Burridge and Wennerberg, 2004; Cernuda-Morollon and Ridley, 2006; Hall, 2005). While GEFs and GAPs mediate GDP exchange and GTPase activation, respectively, the GDIs inhibit GDP dissociation (Etienne-Manneville and Hall, 2002; Sasaki and Takai, 1998). Three evolutionarily conserved mammalian GDIs (GDIα, β and γ) displaying wide spread expression across species have been identified so far (Adra et al., 1997; Lelias et al., 1993; Scherle et al., 1993; Ueda et al., 1990). Among them, GDIα, which is the most extensively studied, is ubiquitously expressed, while GDI β and GDI γ display some tissue specificity of expression (DerMardirossian and Bokoch, 2005; Dovas and Couchman, 2005; Olofsson, 1999; Sasaki and Takai, 1998). The GDIs negatively regulate Rho, Rac and Cdc42 activity through different modes of action including: a. direct inhibition of GDP dissociation, b. extracting the Rho GTPases from the membrane and preventing them to localize to the membrane, and c. interacting with GTP-bound Rho GTPases and preventing GTP hydrolysis and GEF interaction (DerMardirossian and Bokoch, 2005; Dovas and Couchman, 2005).
In this study we have transgenically overexpressed bovine RhoGDIα (which targets Rho, Rac and Cdc42) in the mouse lens epithelium and fibers to explore and understand the role of Rho, Rac and Cdc42 activity in lens epithelial proliferation, and fiber cell elongation and differentiation. This study unveils the distinct effects of impairing the function of Rho GTPases on lens growth, differentiation and fiber cell organization, and demonstrates a critical role for the Rho GTPases in lens fiber cell migration, elongation and survival.
Materials and Methods
Generation of transgenic mice
In this study we used a well-characterized, lens-specific δ1-enhancer/αA-crystallin (δenh/αA) fusion promoter to drive the expression of a bovine RhoGDIα transgene in lens epithelium and fibers (Xie et al., 2006). The bovine RhoGDIα cDNA (614bp, entire coding sequence) obtained from Yoshimi Takai (Fukumoto et al., 1990) was inserted between the rabbit β-globin intron and the human growth hormone (hGH) polyadenylation signal sequences (Fig. 1B). The resultant plasmid with transgene insert (3.36 kb) was excised, purified and injected into the pro-nucleus of B6SJLF1/J mouse embryos (work performed at Duke University Transgenic Facility). Pups were screened for transgene integration into genomic DNA by PCR analysis, using an αA-crystallin promoter-specific forward primer (CCGAGCTGAGCATAGACATT) and bovine RhoGDIα specific reverse primer (CCGGAAGGAGATTTTTATCCGAT). These founder mice were subsequently crossed into a C57/BL6 background to generate the transgenic progeny used in this study. All animal procedures were conducted in accordance with the Association for Research in Vision and Ophthalmology (ARVO) statement for the use of animals in ophthalmic and vision research under an approved Duke University institutional animal protocol.
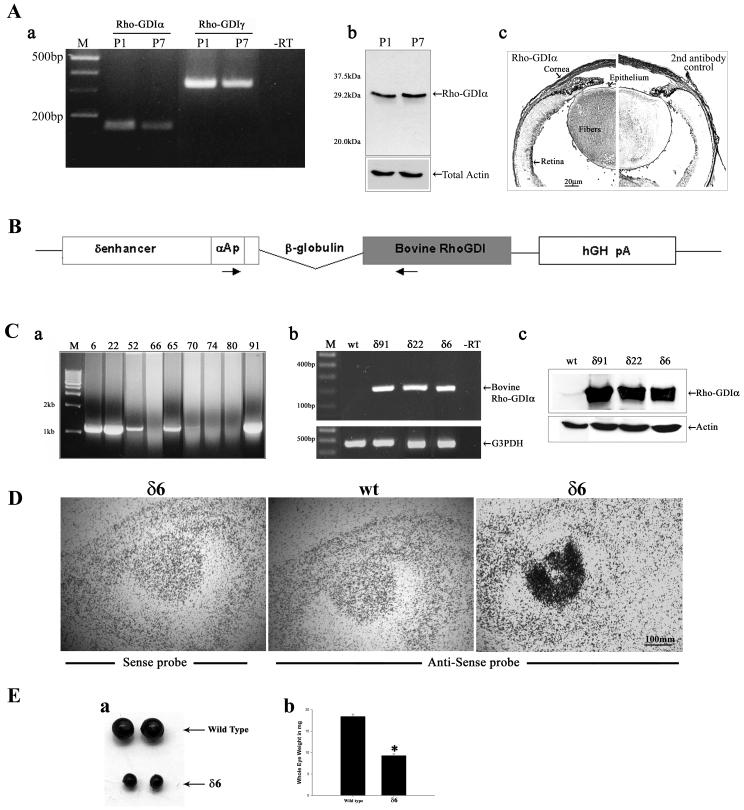
Fig.1.
Constitutive expression and distribution of RhoGDIα in the mouse lens, design of bovine RhoGDIα transgene construct, transgene expression, and ocular phenotype. A. Constitutive expression and distribution of RhoGDIα in P1 and P7 mouse lenses. a. RT-PCR analysis of RhoGDIα and RhoGDIγ expression in P1 and P7 mouse lens; b. Immunoblot analysis of RhoGDIα in P1 and P7 mouse lens and c. Immunohistochemical localization of RhoGDIα in P1 mouse lens. The right half of the panel c depicts the background staining detected using secondary antibody alone B. Schematic diagram of transgenic vector showing insertion of a bovine RhoGDIα coding sequence under the chimeric promoter that contains the mouse αA-crystallin promoter (αAp) linked to the chick δ1-crystallin lens enhancer (δ-enh). A polyadenylation signal sequence from the human growth hormone gene (hGH pA), and rabbit β-globulin intron sequences were added at the 3’ and 5’ends of the RhoGDIα cDNA, respectively. Locations of the primers used for genotying by PCR are shown with arrows. C. Bovine RhoGDIα transgene insertion, expression and distribution in the transgenic mice. a. PCR screening of transgenic and nontransgenic mice using tail DNA, b. Confirmation of bovine RhoGDIα expression in the transgenic mouse lens by RT-PCR analysis, and c. Immunoblotting analysis using anti-RhoGDIα monoclonal antibody to confirm increased levels of total RhoGDIα (both transgenic and constitutive) D. In situ hybridization analysis of bovine RhoGDIα transgene expression in the transgenic mice and note that expression is localized specifically to the lens tissue. E. Transgenic eyes derived from one month-old mice were much smaller in size compared to WT littermates (a) and the wet weights of the Tg eyes were significantly reduced (>50% with P<0.01) compared to those from WT mice (b).
Transgene copy number of each line was determined by quantitative real-time PCR (Q-RTPCR) analysis by following the Roche LightCycler quantification protocol (Technical note No. LC10) using the Bio-Rad iCycler iQ detection system (Bio-Rad, Hercules, CA). The following bovine RhoGDIα-specific forward and reverse oligonucleotide primer sets were used in real-time quantitative PCR analysis: CTG GAC AAG GAC GAC GAG AGT C; CCG GAA GGA GAT TTT TAT CCG AT, to yield a product of 230bp. Concentrations of the bovine RhoGDIα PCR product of one of the Tg lines (δ6) were translated into number of copies per μl of mole DNA as per the Roche Applied Science protocol. This sample was serially diluted to obtain a standard graph ranging 102-109 copies/μl of DNA. Real-time PCR reactions of standards and experimental specimens were carried out simultaneously and the number of copies of Rho GDIα DNA within each experimental sample was then estimated using a standard curve and expressed as copies/μl of mole DNA.
Histological analysis
Embryonic heads (E11.5 and E14.5) and whole eyes (neonatal) of Tg and wild type (WT) littermate mice were fixed in 50 mM cacodylate buffer (pH 7.2) containing 2.5% glutaraldehyde, 4% sucrose and 2 mM CaCl2 for 2 hours, and transferred to 10% buffered formalin. The specimens were subsequently embedded in glycol methacrylate, and 2-μm sections were cut and stained with hematoxylin and eosin (H&E). Micrographs were captured using a Leica TCS NT Microscope.
Immunofluorescence
Cryostat sections (7 μm, sagittal and equatorial planes) were obtained from whole eyes of both Tg and WT animals as described earlier by us (Maddala et al., 2001). Air dried sections were initially treated with image iT™ FX signal enhancer for 30 minutes, rinsed with 0.3% Triton X-100 phosphate buffered saline (PBST), and blocked with buffer containing 5% BSA and 5% goat serum in PBST, for 1 hour at room temperature. Tissue sections were then incubated overnight at 4°C with the respective polyclonal antibodies raised against β-catenin (from Sigma-Aldrich, MO), aquaporin-0 (MIP-26) (kind gift from Dr Sam Zigler), phospho αB-crystallin (Affinity Bioreagents, Golden, CO) or Connexin-50 (from Alpha Diagnostics, San Antonio, TX), all used at a dilution of 1:1000. These sections were washed with PBST and subsequently incubated with Alexa Fluor® 488 goat anti-rabbit secondary antibody (Invitrogen, Carlsbad, CA), for 2 hours at room temperature. After thorough washing with PBST, sections were cover slipped and mounted using 50% glycerol, for viewing under a Zeiss LSM 410 confocal fluorescence microscope. For actin staining, the pre-blocked sections were labeled with phalloidin conjugated with tetra rhodamine isothiocyanate (TRITC) (500 ng/ml; Sigma-Aldrich), as described above.
Immunostaining of RhoGDIα in lens paraffin sections was performed with standard protocol using anti-RhoGDIα polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and Vectastain Universal Quick Kit (Vector Labs, Burlingame, CA). Staining was developed using 3, 3′-diaminobenzidine (DAB) as a substrate according to the manufacturer’s instructions.
Immunoblotting
To determine the expression of RhoGDIα protein in Tg lenses, lens tissue (4-6 pooled) from both the WT and the Tg mice was homogenized in 20 mM Tris buffer, pH 7.4 containing 1mM sodium orthovanadate, 0.2 mM EDTA, 0.2 mM PMSF, 0.1 M NaCl, 50 mM NaF, aprotinin (25 μg/ml), leupeptin (25 μg/ml), and centrifuged at 800x g for 10 minutes at 4° C. Protein concentration was estimated in supernatants by the Bradford method (Bradford, 1976). Equal amounts of lens protein were separated on SDS-PAGE (Sodium dodecyl sulphate-polyacrylamide gel electrophoresis) gels with 12.5% acrylamide, followed by electrophoretic transfer to nitrocellulose membrane. Membranes were blocked over night with 3% milk protein, and then incubated with RhoGDIα monoclonal antibody raised against the intact human protein sequence (BD Transduction laboratories, NJ). Immunoblots were developed by enhanced chemiluminescence (ECL), and scanned densitometrically for analysis using NIH Image software. Similarly, crystallin protein profiles were analyzed in whole lens tissue homogenates, which were prepared from neonatal (P1) WT and Tg mouse lenses as mentioned above. αA and αB-crystallin antibodies were obtained from Joe Horwitz and the rest of the antibodies were from Samuel Zigler. The 800xg lens supernatants were also utilized to quantify the levels of total Rho, Rac and Cdc42 by immunoblot analysis using antibodies raised against anti-RhoA (rabbit polyclonal, Santa Cruz Biotechnology, Santa Cruz, CA), anti-Rac1 (mouse monoclonal, Upstate Biotechnology, Lake Placid NY) and anti-Cdc42 (rabbit monoclonal, Cell Signaling, Danvers, MA).
Separation of membrane rich and soluble lens fractions
To analyze the distribution profile of Rho, Rac and Cdc42 GTPase in membrane rich and cytosolic fractions, whole lenses (P1) from both WT and Tg mice were dissected free of other tissue. The membrane rich and soluble fractions were prepared from pooled samples (4-6 lenses per sample) as described earlier (Maddala and Rao, 2005). Protein concentration was estimated in both the soluble and membrane fractions by the Bradford method (Bradford, 1976). To determine distribution of Rho, Rac and Cdc42 GTPases in the membrane-rich versus soluble fractions, equal amounts (60 μg) of lens soluble (100, 000 x g supernatant) and membrane (100, 000 x g pellet) protein fractions were resolved on SDS-PAGE (12.5% acrylamide) and immunoblotted β-actin expression, which was found to be unaltered in the Tg lenses, was also probed using monoclonal antibody (Sigma, Saint Louis, MO), to confirm loading equivalence. These lens membrane-rich fractions were also used for immunoblotting analysis of phospho-αB crystallin (Affinity Bioreagents, Golden. CO), aquaporin-0 and intermediate filament protein CP-49. CP-49 antibody was a kind gift from Roy Quinlan.
In situ hybridization
Tissue cryosections (7 μm sagittal sections) derived from the eyes of neonatal (P1) and embryonic whole heads (E17.5) of Tg and WT littermate mice were hybridized with 35S-labeled riboprobes. Transgene-specific riboprobes were generated from Bluescript SK- plasmid (Stratagene, La Jolla, CA) into which human growth hormone (hGH) polyA sequences (see Fig.1B) were subcloned at the EcoRI site. Using M13 primers, the promoter regions inclusive of multiple cloning site regions were amplified, these PCR products were gel purified and used to generate the sense and the anti-sense probes by in vitro transcription using T3 or T7 RNA polymerases (Promega, Madison, WI) respectively. Sections stored at -80 °C were post fixed in 3% paraformaldehyde in phosphate-buffered saline (PBS; pH 7.0), acetylated, dehydrated in an ascending ethanol series, and air-dried. These processed sections were then used for hybridization with either anti-sense or sense 35S-labeled probes (1 × 106 cpm) by a standard method.
BrdU incorporation
Timed pregnant mothers at 15.5 day post-conception derived from all three different transgenic lines, were injected with bromo-deoxy-uridine (BrdU) at a concentration of 100 μg/gram body weight and were sacrificed after two hours of incorporation. Heads of the E15.5 fetuses were fixed for cryosectioning according to the procedure mentioned above. After genotyping, 7 μm serial sections were cut and used for further analysis. To denature the DNA, air dried cryosections (from WT and Tg) were incubated in 2N HCL with 0.5% Triton X100 for 30 minutes at room temperature and neutralized with 0.1M sodium tetra borate pH 8.5. After several washings in PBS, tissue sections were blocked (2% BSA in PBS containing 0.5% Triton X100) for 30 minutes. For direct labeling of BrdU, Fluorescein (FITC)-tagged anti-BrdU antibody from BD Biosciences (Palo Alto CA), was applied onto the sections and incubated for 30 minutes at room temperature. After excessive washing with PBS, these sections were counterstained with propidium iodide, and images were recorded using a fluorescence microscope (Zeiss Axioplan-II). Total number of nuclei and the percent BrdU positive cells in the lens epithelium and fiber mass of Tg and WT mice were manually counted and assessed for statistical significance by student t-test. An average of 6-8 individual lens sections derived from two different mice was analyzed per each Tg line.
TUNEL assay
In situ terminal transferase dUTP nick end labeling (TUNEL) staining was performed using an ApopTag Plus Fluorescein kit (Chemicon, Temecula, CA) to evaluate and compare apoptotic cell death in Tg mouse lens sections verses those from WT littermates. Lens cryosections (7 micron sagittal sections), from both P1 and E15.5 WT and Tg animals were mounted on gelatin-coated glass slides, fixed first in 1% paraformaldehyde and then post-fixed in pre-chilled ethanol:acetic acid (2:1). Multiple tissue sections were incubated with working strength TdT enzyme (terminal deoxynucleotidyl transferase), which catalytically adds digoxigenin-nucleotides to the fragmented DNA. Tissue sections were then incubated with fluorescein-tagged anti-digoxigenin antibody. TUNEL labeled sections and were further co-stained for nuclei with propidium iodide. Apoptotic cells were scored using a fluorescence microscope (Zeiss Axioplan-II).
Results
Increased expression of RhoGDIα in the ocular lens leads to microphthalmic eyes
We examined for the constitute expression of RhoGDIα in neonatal (P1) and postnatal (P7) WT mouse lenses by RT-PCR and western blot analysis. Both P1 and P7 lenses showed easily detectable levels of RhoGDIα expression by these analyses (Fig. 1A, panels a, b). Immunohistochemical analysis further reveals that RhoGDIα is distributed throughout the lens including epithelium, elongating and differentiating lens fibers with stronger staining in the lens fibers relative to the epithelium (Fig. 1A, panel c). The right and left half in Fig. 1A (panel c) depict the immunostaining of RhoGDIα in mouse lens in the absence and presence, respectively, of RhoGDIα primary antibody. Both P1 and P7 lenses were also shown to express the RhoGDIγ, (Fig. 1A, panel a) which is reported to have preferred expression in the brain (Adra et al., 1997).
Several independent lines of mice expressing the bovine RhoGDIα transgene under the control of a lens-specific chimeric δenh/αA-crystallin promoter were generated (Fig. 1C). The chimeric promoter consisting of a chicken δ1-crystallin gene enhancer element and the mouse αA-crystallin promoter (Fig. 1B) has been shown to drive transgene expression in both lens epithelial and fiber cells (Reneker et al., 2004). Many of the transgene positive founders (Fig. 1C, a) exhibited ocular phenotypes including bilateral microphthalmia with lens opacity. From three independent founders (δ6, δ22 and δ91) displaying marginally different degree of phenotype and carrying different copy numbers of the transgene based on quantitative real-time PCR analysis (5.5 × 108, 3.9 × 108 and 1.2 × 109 copies per μl of mole DNA, respectively), we generated heterozygous progeny by breeding founders back to the C57/BL6 mice, and characterized them in detail. Mice from these three independent lines (δ6, δ22 and δ91) were examined for the expression of RhoGDIα transgene by RT-PCR using sequence specific oligonucleotide primers derived from the sequence of bovine RhoGDIα. As shown in Fig. 1C (panel b), only Tg lenses (pooled samples) yielded a positive PCR product for the bovine specific RhoGDIα. Additionally, immunoblotting analysis of lens homogenates (4-6 lenses pooled per sample) derived from P1 mice from the three different lines (δ6, δ22 and δ91) revealed markedly increased levels of total RhoGDIα protein (both constitute and transgenic) with levels being 9.6, 11.2 and 15.1 -fold higher, respectively, as compared to that noted in WT lenses (Fig. 1C, panel c). Additionally, in situ hybridization of E17.5 and P1 Tg eyes with transgene-specific riboprobe confirmed the lens-specificity of transgene expression, and revealed a distribution pattern that was much higher in lens fibers compared to the lens epithelium (Fig. 1D shows data from a δ6 E17.5 specimen).
All three Tg lines consistently exhibited the lens opacity based on slit lamp microscopy (data not shown). Further, the size of the postnatal Tg eyes (from all three lines) was significantly smaller than the WT littermate eyes, with the eyes derived from one month-old Tg mice showing a 50% or higher reduction in wet weight compared to their WT littermates. Data are shown for the Tg line δ6 (Fig. 1E; a, b).
Increased RhoGDIα expression in transgenic lenses disrupts membrane translocation of Rho GTPases
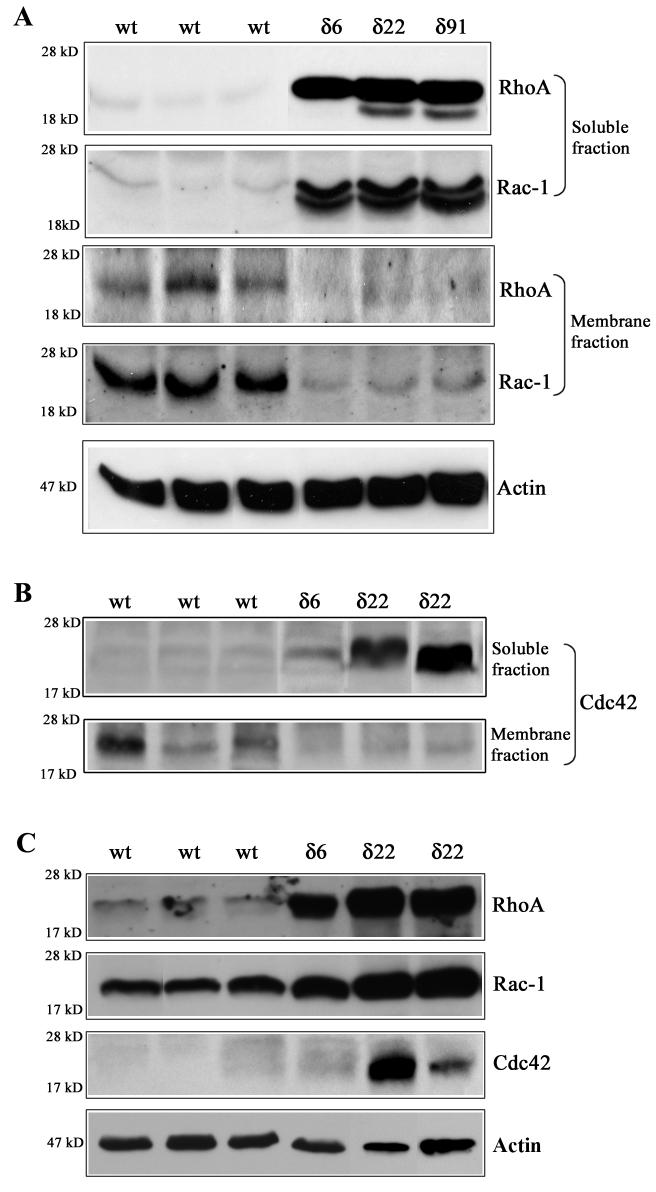
To determine the effects of increased expression of RhoGDIα on the distribution of Rho, Rac and Cdc42 GTPases, equal amounts of protein from lens soluble and membrane-rich fractions obtained from the P1 Tg (δ6, δ22 and δ91 lines) and WT mice were subjected to immunoblotting analysis. In multiple samples from different transgenic lines, higher levels of Rho, Rac and Cdc42 GTPase immunoreactivity were found to be associated with soluble fractions as compared to the WT lenses (Fig. 2A, B). Conversely, lower levels of Rho, Rac and Cdc42 GTPases immunoreactivity were noted in the membrane rich fractions of Tg lenses, relative to WT lenses (Fig. 2A, B). Immunoblots of Rho and Rac GTPases derived from the soluble fractions of the Tg lenses showed two closely migrating immunopositive bands, presumably as a result of accumulation of both isoprenylated and non-isoprenylated GTPases (Fig. 2A). The accumulation of Rho, Rac and Cdc42 GTPases in the soluble fraction of Tg lenses indicates defective membrane translocation very likely due to higher levels of Rho GDIα in the Tg lenses (Fig. 2A, B). Since membrane localization is essential for activity of the Rho GTPases, the defective membrane translocation of Rho GTPases noted in the RhoGDIα Tg lenses is indicative of compromised signaling through the affected Rho GTPase-dependent pathways (Etienne-Manneville and Hall, 2002).
Fig. 2.
Defective membrane translocation, and elevated levels of Rho, Rac and Cdc42 GTPases in RhoGDIα transgenic mouse lenses. Distribution of Rho and Rac GTPases (Panel A), and Cdc42 (panel B) in the soluble and membrane rich fractions of lens tissue obtained from P1 Tg and WT mice. To evaluate the influence of RhoGDIα overexpression on the membrane localization of Rho, Rac and Cdc42 GTPases in the Tg and WT lenses, soluble and membrane protein fractions were isolated from the P1 lenses (pooled samples) and Rho, Rac and Cdc42 protein levels were evaluated by immunoblot analysis using equal amounts of protein. These analyses were carried out in multiple and a representative immunoblot is shown here. The soluble fractions were also immunoblotted for actin to confirm equal protein loading among the different samples. C. Increased levels of Rho, Rac and Cdc42 GTPases in transgenic lens homogenates (800xg supernatant). Lens homogenates (from pooled lenses) derived from the Tg and WT mice were subjected to immunoblot analysis. The same samples used in panel B and C were also immunoblotted for actin to confirm equal protein loading. The Tg line 91 was not included in these analyses.
To further confirm impaired activity of Rho GTPases in RhoGDIα overexpressing Tg lenses, we also performed RhoGDI-Rac interaction assays using recombinant GST-RhoGDI (Peterson et al., 2006). Rho GDIα has been shown to have similar binding and inhibitory activity towards all three Rho GTPases including Rho, Rac and Cdc42 (Olofsson, 1999). Rac1 which is expressed at higher levels than the Rho and Cdc42 in the mouse lens (Fig. 2C) was analyzed as a representative of different Rho GTPases. Unlike the RhoGDI-Rac complexes from wild-type lenses that are clearly immunopositive for Rac, transgenic lens RhoGDI-Rac complexes did not contain detectable levels of Rac GTPase. This observation indicates that most of the Rac in transgenic lenses already exists in the form of RhoGDI-Rac complexes, owing to the higher amounts of RhoGDI present in these lenses, and therefore not being available for interaction with the GST-RhoGDI used as an immunoaffinity reagent in the pull-down assay (please see enclosed supplemental Figure).
Interestingly, the protein levels of total Rho, Rac and Cdc42 were found to be much higher in the 800xg supernatant fractions from transgenic lenses compared to those in WT lenses based on the immunoblot analysis (Fig. 2C). However, this increase in total Rho GTPases protein levels in the transgenic lenses did not translate into elevated levels of free/active Rho GTPases in the RhoGDI-Rac pull-down assays described above (Supplemental Figure).
RhoGDIα overexpression in the transgenic lens disrupts fiber cell migration, elongation and organization
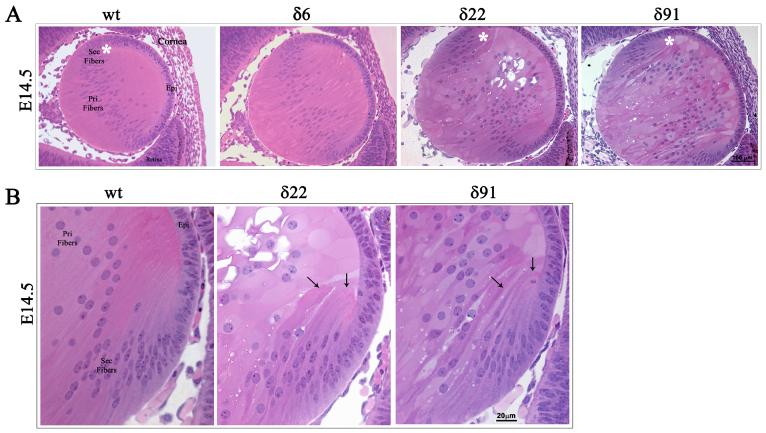
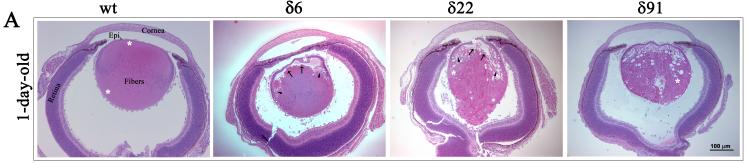
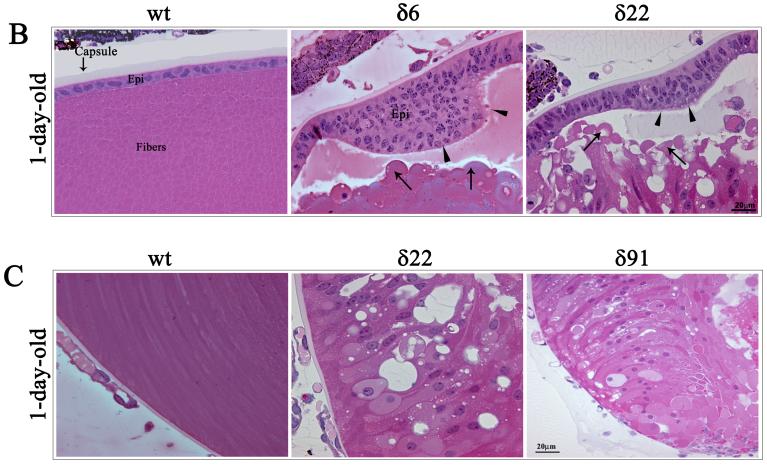
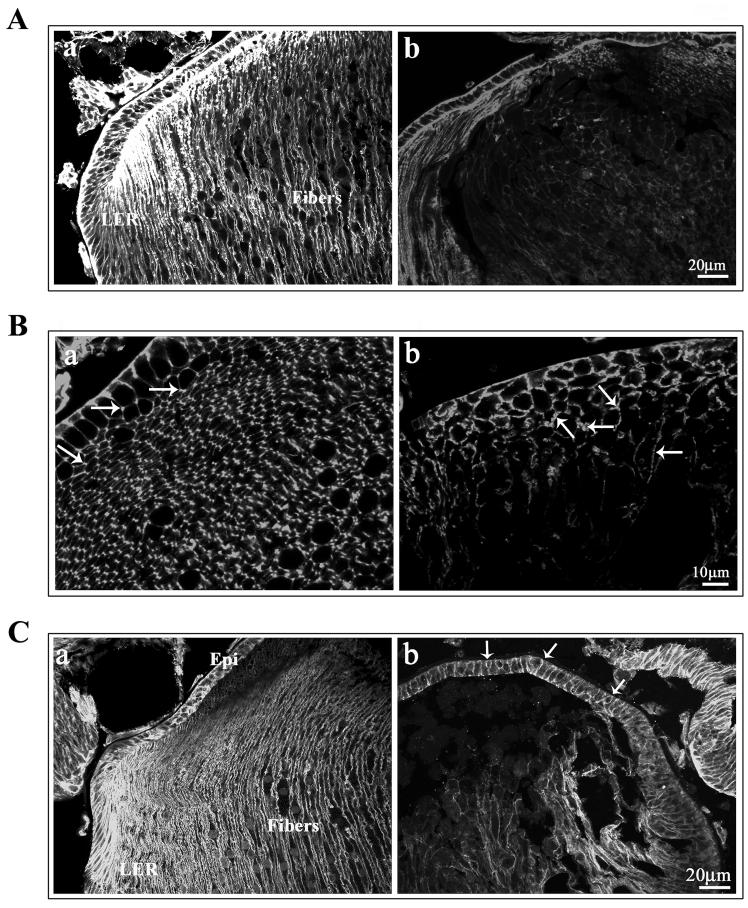
The RhoGDIα Tg lenses derived from three independent lines (δ6, δ22 and δ91) exhibit normal development at E11.5 and are indistinguishable from WT littermates based on the histological analysis (not shown). At E14.5, in the Tg animals (line δ22 and δ91), however, the organization of primary lens fibers exhibits abnormalities, comprising of a swollen fiber cell morphology and vacuolization (Fig. 3A). Fiber cell migration and elongation pattern was found to be defective at the equatorial region, and distinctly different from that observed in WT lenses. Moreover, the secondary fibers cells of Tg lenses do not exhibit the characteristic anterior to posterior stretched symmetry (Fig. 3A and B). In the WT lenses, the anterior tips of migrating and elongating fibers stretch and make contacts with the apical surface of the epithelium and migrate towards the suture line, while the anterior tips of the fiber cells of the Tg lenses in contrast, bend and migrate towards the equatorial epithelium (Fig. 3B indicated with arrows). In Tg line δ6, the E14.5 lenses showed less obvious abnormality. Intriguingly, at E14.5, the Tg lenses from all three lines were found to be slightly bigger in size (by 18% based on mean of 13 Tg lenses from three different lines) than the WT lenses (n=8), with this being much more pronounced in Tg line δ91 (26% larger than the WT), which has relatively a high copy number of the transgene compared to Tg lines δ6 and δ22. At day one (P1), the Tg lenses exhibited gross defects in the lens fiber cell organization in all three lines (Fig. 4A). The anterior tips of the secondary lens fibers fail to elongate and do not make connections with apical surface of the epithelium, and is accompanied by extensive vacuolization at the equatorial region of the lens (Fig. 4A indicated with arrow heads). Additionally, the primary lens fibers in P1 Tg lenses fail to maintain connections with the central epithelium (Fig. 4A and B indicated with arrows). The primary lens fibers appear to retract from the apical surface of the lens epithelium, an effect which might derive from the defective secondary fiber cell elongation and migration. The posterior tips of both the secondary and primary lens fibers in Tg lens appear to extend straight towards the capsule instead of migrating towards the suture line (Fig. 4C). Therefore, the Tg lenses do not exhibit the suture line that we see in the postnatal WT lenses indicating defective fiber cell sliding and migration in the Tg lenses. The Tg lenses from both line 22 and 91 frequently exhibit a ruptured capsule at P1 and postnatal, with lens material leaking into the vitreous cavity (Fig. 4A). Furthermore, unlike in the WT lenses where the primary fiber cells exhibit denucleation, primary lens fibers from Tg lenses contain intact nuclei, indicating defective fiber cell terminal differentiation (Fig. 4C). The epithelium of the P1 Tg lenses frequently exhibits a thickened morphology with multilayered nuclei compared to a single layer of nuclei in the WT lenses (Fig. 4B, indicated with arrow heads). The Tg lenses of one month-old mice from all three lines exhibited ruptured capsules and complete degeneration (data not shown).
Fig. 3. Abnormal lens phenotype in the embryonic RhoGDIα transgenic mice.
A. Hematoxylin and Eosin stained sagittal sections of E14.5 WT and RhoGDIα Tg eyes. The E14.5 Tg lenses from all three lines exhibit a marginal increase in size compared to wild type lenses. The magnification of WT and Tg lenses is the same. E14.5 lenses from Tg lines δ22 and δ91 show abnormal fiber cell morphology and defective secondary fiber cell organization. B. Higher magnification of E14.5 Tg lenses (white asterisks in panel A figures indicate the magnified area shown in panel B) at the equatorial region reveals defective fiber cell orientation and migration (indicated with arrows).
Fig. 4. Abnormal lens phenotype in the neonatal RhoGDIα transgenic mice.
A. Hematoxylin and Eosin stained sagittal sections of P1 WT and RhoGDIα Tg eyes. P1 Tg lenses from all three lines reveal gross morphological changes especially in the lens fibers including defective lens fiber cell attachment with lens epithelium, accumulation of vacuoles (arrow heads), blunted fiber cell anterior tips (arrows) and ruptured posterior capsule. B. Abnormal morphology of lens epithelium in Tg lenses with multilayered nuclei and thickened epithelium (arrow heads). Globulization of secondary lens fibers and detachment of primary lens fiber cell anterior tips from the apical surface of lens epithelial cells in the transgenic mice (indicated with arrows). C. While in WT, the posterior lens fibers migrate towards the suture line, in the Tg lenses the fibers are completely disorganized, displaying an abnormal morphology and accumulate of nuclei. White asterisks in panel A figures indicate the magnified area shown in panel B and C.
Effect of RhoGDIα overexpression on lens cell proliferation, differentiation and survival
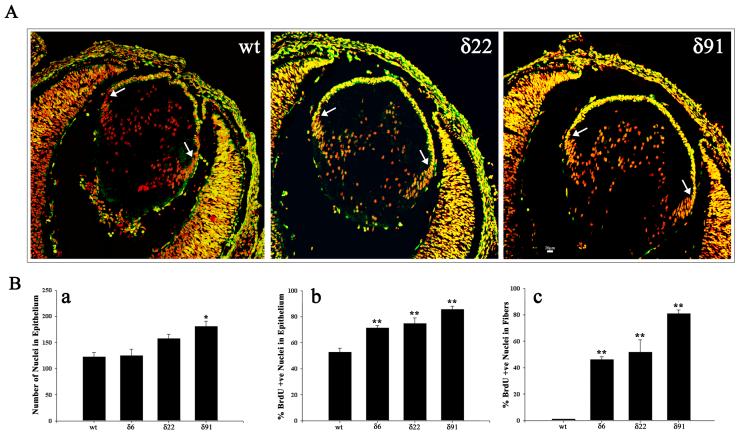
To determine the effects of RhoGDIα overexpression on cellular proliferation in the developing lens, we conducted in vivo BrdU incorporation experiments by injecting BrdU into pregnant animals (all three Tg lines) and examining E15.5 embryos for BrdU incorporation using a fluorescein-conjugated anti-BrdU monoclonal antibody. Analysis of BrdU immunolabeling using whole head tissue cryosections derived from both the Tg (δ6, δ22 and δ91) and WT littermate E15.5 embryos, revealed BrdU incorporation into the lens epithelium (Fig. 5A). However, when compared to the WT lenses, the entire central epithelium (region between the right and left equatorial/germinative zones indicated with arrows) of the Tg lenses showed increased BrdU labeling (Fig. 5A; Tg 22 and Tg 91). Further, some of the lens fibers of Tg lenses also showed an increased BrdU labeling compared to respective WT lens fiber cells. Examination of multiple lens sections (6-8) derived from different Tg and WT embryos revealed a consistent and significant increase in BrdU incorporation into the central epithelium (Fig. 5B, b) and in primary fiber cells (Fig. 5B, c) of Tg lenses, indicating abnormal proliferation of lens epithelial cells overexpressing RhoGDIα (Fig. 5B). Figure 5B (panel a) shows an increased total number of nuclei in the Tg lens epithelium as compared to the WT lens epithelium. Since the various WT lenses showed identical values, data derived from WT littermate of Tg line 22 was given as a representative (Fig. 5A, B).
Fig. 5. Abnormal BrdU incorporation in RhoGDIα transgenic lenses.
A. In vivo BrdU incorporation was performed to evaluate lens epithelial cell proliferation status in Tg mice. In WT E15.5 lenses, the BrdU-labeling was localized (bright yellow fluorescence) to the central and equatorial regions of the epithelium (anterior region between the two white arrows). However, compared to WT lenses, in the Tg lenses (δ6, δ22 and δ91), the entire central epithelium revealed intense BrdU positive labeling (bright yellow fluorescence). Additionally, most of the nuclei in the fiber cells were positive for BrdU incorporation in the Tg lenses. Lens sections were co-labeled with propidium iodide to localize the cell nucleus (red fluorescence). Representative images from the Tg line δ22 and δ91 are shown in this figure. B. Quantitative differences in BrdU incorporation in the lens central epithelium (panel b) and differentiating fibers (panel c) between WT and Tg mice. Compared to WT lenses, % BrdU incorporation was found to be significantly higher in the central epithelium (panel b) and fibers (panel c) in transgenic lenses. Panel a, indicates changes in the total number of nuclei in central epithelium between WT and Tg lenses. * P<0.05, ** P>0.01; 6-8 lens sections were analyzed from two independent mice. Since the various WT lenses showed identical values, data derived from WT littermate of Tg line 22 was given as a representative (Fig. 5A, B).
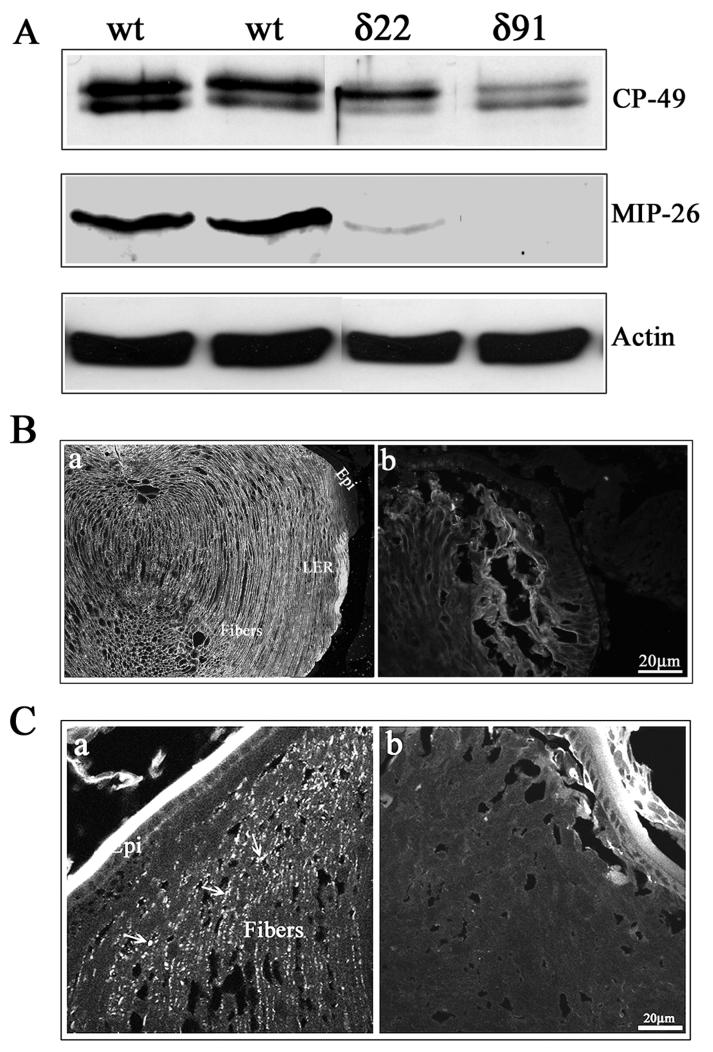
To examine the effects of increased RhoGDIα expression on lens differentiation-induced expression profiles of crystallins in the lens, we evaluated crystallin protein levels in P1 lens total homogenates by immunoblotting analysis. Crystallins are the most abundant lenticular proteins whose expression is induced during lens epithelial cell differentiation, owing to which, differentiated lens fibers display high levels of crystallins relative to the lens epithelium (Piatigorsky, 1981). We determined the levels of αA, βB2 and γ-crystallins in the total homogenates of Tg and WT lenses of P1 mice using polyclonal antibodies. Crystallin profiles were found to be very similar between the Tg and WT lenses (data not shown). Western blotting and immunofluorescence techniques were additionally used to evaluate the levels and distribution of aquaporin-0, phakanin (CP-49) and connexin-50, which are lens fiber specific proteins whose expression is initiated specifically during lens fiber cell differentiation (Piatigorsky, 1981). The levels of Aqaporin-0, a water channel protein which is also identified as major intrinsic protein- 26 (MIP-26) were markedly decreased to almost undetectable levels in P1 Tg lenses compared to the WT lenses, based on the immunoblot analysis (Fig. 6A). Similarly, immunolabeling of Tg lens cryosection with anti-MIP 26 antibody revealed very weak staining in the lens fibers (Fig. 6B, panel b) relative to very specific and intense immunostaining along the fiber cell membrane in the WT lenses (Fig. 6B, panel a ). The levels of phakanin, the lens specific beaded filament protein were also decreased in the P1 Tg lenses compared to WT lenses (Fig. 6A). Additionally, immunofluorescence-based localization of Connexin-50, a lens fiber-specific gap junction protein, revealed specific and punctate staining pattern localizing along the lens fiber cell membrane in WT lenses (Fig. 6C; a, indicated with arrows). In the Tg lenses, however, the lens fibers did not exhibit any specific immunopositive staining for connecxin-50 (Fig. 6C, panel b). These observations collectively indicate impairments in fiber cell membrane make-up, organization and very likely function, in the Rho GDIα Tg lens fibers.
Fig. 6.
Defective organization of water channel and gap junctional proteins in the RhoGDIα transgenic lens fibers. A. P1 Tg lens homogenates (800xg supernatants) showed decreased levels of fiber-specific beaded filament protein (CP-49, also called phakanin), water channel protein Aquaporin-0 (also called MIP-26) compared to WT lenses, as determined by western blot analysis. Actin, which was immunoblotted to confirm equality of protein loading, indicated no differences between the WT and Tg specimens. B. Immunofluorescence distribution of aquaporin-0 in P1 WT and Tg lens cryosections. While immunolabelling for aquaporin-0 shows a fiber cell specific distribution along the cell membrane in WT lenses, a much weaker immunostaining that was not specifically localized to the lens fiber cell membrane, was noted in the case of Tg lenses. C. Immunofluorescence distribution of connexin-50, a lens fiber-specific gap junction protein in Tg and WT lenses. Similar to aquaporin-0, WT lenses reveal a specific and punctuate immunopositive staining pattern for connexin-50 distributed along the fiber cell membrane (panel a, indicated with arrows), while there was no detectable immunopositive labeling for connexin-50 in the Tg lenses (b). The lens capsule exhibits a non-specific bright staining in both WT and Tg specimens.
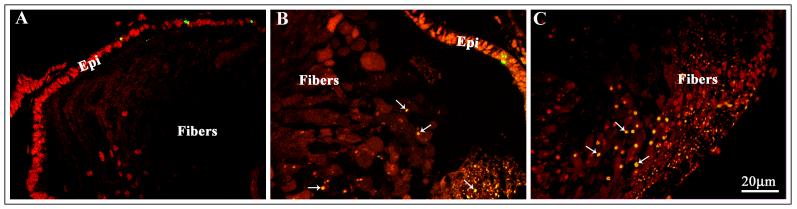
The RhoGDIα Tg lenses at embryonic stage (E14.5) were found to be slightly bigger in size compared to the WT littermate lenses (Fig. 3A). However, neonatal and postnatal Tg eyes and lenses were found to be much smaller than the WT (Fig.2A and 4A). To determine whether aberrant cell survival status was responsible for the decreased eye and lens size in the Tg mice, we performed the TUNEL assay in lens sections derived from P1 mice using the fluorescein-tagged anti-digoxigenin antibody (Fig. 7). While WT lens specimens showed no significant positive TUNEL labeling (Fig. 7A) both in epithelium and fibers, the Tg lenses revealed increased TUNEL positive staining, especially in the degenerative fiber cells. As shown in Fig. 7B and C, the P1 fiber cells of Tg lenses are grossly degenerated, disorganized and the nuclei is scattered throughout the fiber cells. Further, these same tissue sections exhibited extensive TUNEL positive staining (indicated with arrows) in the lens fiber mass compared to WT lenses. To localize the nuclei, these lens sections were co-stained with propidium iodide and in the superimposed photographs of the TUNEL staining and propidium iodide staining, the bright yellow fluorescence indicates apoptotic cell death (indicated with arrows). The corresponding lens posterior region from WT lenses did not exhibit positive propidium iodide labeling of nuclei (not shown). The photograph in Fig. 7 is a representative of the multiple analyses with the same degree of TUNEL positive staining in the Tg lenses. Compared to P1 Tg lenses, the E15.5 Tg and WT lenses did not reveal any difference in TUNEL staining.
Fig. 7. Increased apoptotic cell death of the lens fibers in the RhoGDIα transgenic eyes.
P1 WT and Tg lens cryosections were examined for apoptotic cell death by detecting the digoxigenin-nucleotides using a fluorescein-tagged anti-digoxigenin antibody. These same lens sections were also co-stained with propidium iodide to localize nuclei. While WT lens sections (A) revealed no TUNEL positive cells in the fiber cells, degenerating lens fibers of Tg lens showed increased TUNEL positive immunostaining in the central (B) and posterior (C) regions (indicated with arrows). Epithelium from both WT and Tg lenses showed sporadic TUNEL positive cells but with no significant difference between the two types of lenses. A representative image is shown based on multiple analyses.
Disrupted organization of actin cytoskeleton and cell-cell adhesions in RhoGDIα overexpressing mouse lens
Regulation of actin cytoskeletal organization, cell-ECM and cell-cell interactions constitute the by far most well-understood cellular activity of Rho GTPases (Burridge and Wennerberg, 2004; Etienne-Manneville and Hall, 2002). Since overexpression of the RhoGDIα is expected to impair membrane localization as shown in Fig. 2B and is consequently expected to impair Rho GTPases activity, we examined the status of actin filament organization and distribution of β-catenin, a cadherin interacting cell adhesion protein in the RhoGDIα Tg lenses, by immunofluorescence staining. Based on phalloidin-rhodamin staining, distribution of the actin filament network, appeared to be uniform in the epithelium and in the differentiating fibers of the WT P1 lens cryosections (both sagittal and equatorial planes Fig. 8A and B, respectively). Actin filaments are localized prominently along the cell membrane of both epithelial (basal to apical) and fiber cells (from anterior tips to posterior tips, in the sagittal sections Fig. 8A; a) in crytosections from WT P1 lenses. In contrary, the phalliodin staining was diminished markedly and displayed a less than distinct distribution along the lens fibers, relative to that noted for WT lenses (panel b in Fig. 8A and B). Labeling of filamentous actin in the WT lens sections derived from equatorial plane showed a uniform and clustered localization at the angles along the short side of the hexagonal fiber cells (indicated with arrows, Fig. 8B; panel a) consistent to earlier reported observations (Lee et al., 2000; Lo et al., 1997). On the other hand, in the Tg lenses, the fiber cells were organized in distorted manner and did not exhibit the typical hexagonal morphology (Fig. 8B; b). Importantly, the organization of the filamentous actin is not localized to the angles of the fiber cells, being spread throughout the plasma membrane instead (Fig. 8B; b indicated with arrows). The diminished staining of phalloidin in the Tg lenses was confirmed as not being related to the changes in the actin content. Immunoblot analysis of both the Tg and WT lenses for the total actin content showed no significant difference between the two groups (data not sown) indicating the diminished staining could be related to defective actin filament organization and polymerization in the Tg lenses.
Fig. 8.
Defective organization of actin filament and adherens junction-associated β-catenin in the RhoGDIα transgenic lenses. Sagittal (A) and equatorial (B) plane cryosections from P1 WT and Tg lenses were stained for filamentous actin with rhodamin-phalloidin, and fluorescence staining images were captured with a confocal microscope. While the actin filament network is distributed uniformly in the WT lens epithelium and fibers along the cell membrane in the tissue sections derived from the sagittal plane (A; panel a), in the Tg lens fibers (A; panel b), actin distribution is distorted with irregular organization and a much reduced staining. Images viewed from the equatorial plane of the WT lens sections (B; panel a) revealed a uniform and clustered localization of actin filament at the angles of the short side of the hexagonal fiber cells (indicated with arrows), in the lens epithelial cells, it is distributed from basal to apical side along the cell membrane. On the other hand, in the Tg lenses (B; panel b), the fiber cells exhibit an asymmetric and distorted cell morphology, and actin filaments do not exhibit the atypical clustered localization at the angles of the hexagonal fiber cells, being distributed uniformly along the fiber cell membrane (indicated with arrows). C. Distribution of β-catenin in P1 WT and Tg lenses. The sagittal P1 lens cryosections immunostained with anti-β-catenin polyclonal antibody in conjunction with FITC-conjugated secondary antibody showed distribution of β-catenin at the intercellular junctions of the epithelial cells, and in fibers it is distributed along the fiber cell membrane, spreading from anterior to posterior tips (panel a). In the epithelium of Tg lenses (panel b), β-catenin distribution was found to be similar to that of WT lenses, localizing to the intercellular junctions (arrows). In the fibers, however, its distribution was found to be distorted and did not show uniform localization along the fiber cell membrane and exhibited a much weaker staining compared to WT lenses.
Immunostaining analysis for β-catenin revealed its distribution along the intercellular junctions of the epithelial cells, and along the fiber cell lateral membrane in the WT lenses (Fig. 8C; panel a). The epithelium, the newly differentiating, and secondary fibers of the WT lens in the cortical region exhibited very strong and specific positive staining for β-catenin along the fiber cell membrane (Fig. 8C; a). While the epithelium of Tg lenses revealed a normal distribution of β-catenin localizing to the cell junctions (indicated with arrows), (Fig. 8C, b), the lens fibers, especially the disorganized fibers, exhibited markedly diminished staining for β-catenin, which additionally do not localize along the fiber cell lateral membrane strongly implying abnormalities of cell adhesive activity in the RhoGDIα overexpressing Tg lenses (Fig. 8C; b).
Robust activation of αB-crystallin phosphorylation in the RhoGDIα overexpressing mouse lens epithelium
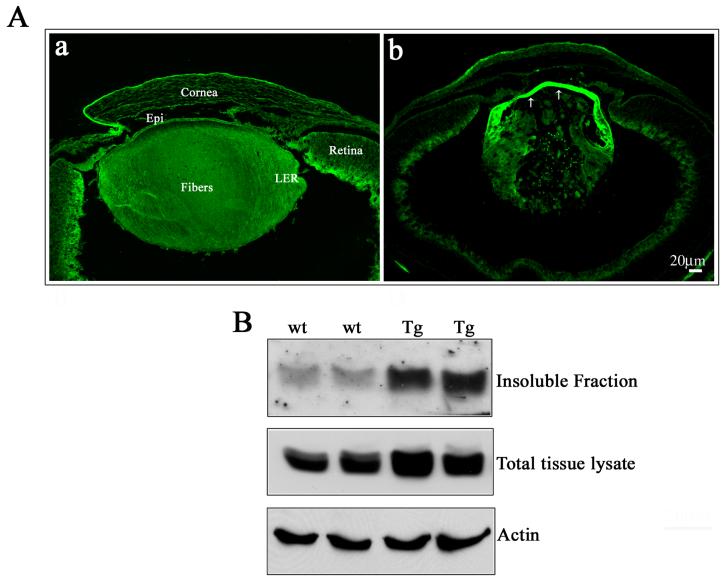
αB-crystallin, a small heat shock protein and an abundant lens protein binds to actin and other cytoskeletal proteins, is thought to possess chaperone-like activity and participate in membrane organization (Horwitz, 2003). Further, in migrating lens epithelial cells, αB-crystallin has been shown to localize to the leading edges in a phosphorylation-dependent manner, and to co-localize with abl-kinase interacting proteins (Abi-1 and Abi-2) and WAVE, which are regulators of actin dynamics (Maddala and Rao, 2005). To determine the effects of overexpression of RhoGDIα on the distribution of phosphorylated αB-crystallin, we immunostained the P1 lens sections of both Tg and WT with Ser-59 phosphospecific anti-αB-crystallin polyclonal antibody (Fig. 9A; panels a, b). While phospho-αB crystallin was spread uniformly throughout the lens including the epithelium and fibers (Fig. 9A; a) of WT lenses, Tg lenses in contrast, exhibited an intensely staining epithelium (Fig. 9A; b indicated with arrows). Both the equatorial and central epithelium of the Tg lenses revealed a very intense and increased staining for Ser-59 phospho αB-crystallin, relative to that observed in the WT lens. On the other hand, the degenerating fiber mass of the Tg lens did not show much difference. Western blot analysis of total lens lysate (800xg supernatant) and membrane fractions (100,000xg pellet) of the lens tissue derived from the Tg and WT demonstrated increased levels of phospho-αB in both these fractions in the Tg lenses as compared to the WT lenses (Fig. 9B).
Fig. 9. Increased αB-crystallin phosphorylation in the RhoGDIα transgenic lens epithelium.
A. P1 WT and Tg lens cryosections immunostainined with a Ser-59 phosphospecific αB-crystallin antibody revealed the presence of phosphorylated αB-crystallin in the epithelium and fibers cells (a). However, while distribution of phosphorylated αB-crystallin was uniform between the epithelium and fiber cells of WT lenses, the Tg lenses (b), exhibited a very intense staining for phosphorylated αB-crystallin throughout the epithelium, including at the equatorial and central regions (arrows). A representative photograph is presented in this figure based on multiple analyses using lens sections derived from the different Tg lines. B. Transgenic lens total (800x supernatants) or insoluble fractions (100,000xg pellets) immunoblotted with phosphospecific αB-crystallin antibody showed a significant increase in the levels of phosphorylated αB-crystallin, both in total homogenate and membrane fractions compared to the WT lenses. Actin was probed in the same samples to confirm loading equivalence for protein.
Discussion
This study reveals that overexpression of RhoGDIα during development disrupts lens morphogenesis and illuminates a critical requirement for the Rho, Rac and Cdc42 GTPase activity in regulating lens fiber cell migration, elongation, organization and survival. The most obvious and early effect of Rho GDIα overexpression is that the secondary lens fibers fail to migrate and elongate properly, owing to which, the lens fibers do not reach the lens epithelium or make cell-cell contacts with epithelial cells, lack symmetry and fail to form lens sutures. Furthermore, the RhoGDIα overexpressed Tg lens fibers exhibit disruption in organization of fiber cell gap junctions, water channels, and actin cytoskeletal organization and cell-cell junctions.
Lens morphogenesis and shape depend mainly on lens epithelial cell proliferation, migration, polarization, elongation and differentiation, processes known to be regulated spatially and temporally by various external factors (Chow and Lang, 2001; Lovicu and McAvoy, 2005; Piatigorsky, 1981). However, the mechanistic bases underlying these cellular processes and the role(s) of different intracellular pathways in these events are largely unknown. The Rho GTPases including Rho, Rac and Cdc42, have been shown to play a crucial role in controlling various cellular activities including cell migration, polarity, trafficking, proliferation, differentiation and survival (Burridge and Wennerberg, 2004; Cernuda-Morollon and Ridley, 2006; Etienne-Manneville and Hall, 2002). These processes are mediated via the direct involvement of the Rho GTPases in regulation of actin cytoskeletal organization, actin polymerization, cell adhesions, cell cycle progression and transcriptional activation (Burridge and Wennerberg, 2004; Hall, 2005). However, these conclusions are derived largely based on cell culture studies, and our knowledge regarding the role of the Rho GTPases in organ morphogenesis and development is limited (Maddala et al., 2004; Togawa et al., 1999; Wei et al., 2002). Our observations in this study unveil the vital role played by the Rho GTPases in lens fiber cell migration and elongation, and mouse lenses overexpressing RhoGDIα, which is an in vivo inhibitor of Rho GDP dissociation, exhibited disruptions of lens fiber cell migration and elongation, and abnormal changes in cell shape and organization.
Intriguingly, at E14.5, the RhoGDIα Tg lens size appears to be actually larger in size compared to WT lenses. Although this change appears to be marginal (18%), it was found to be consistent among the different Tg lines (Fig. 3A). Interestingly, in the in vivo BrdU incorporation experiments, the Tg E15.5 lenses showed increased incorporation (75 to 85%) in the central epithelium as compared to the WT littermates, where only 50% cells revealed positive BrdU labeling (see Fig. 5A and B). Further, the fiber cells which normally exit the cell cycle by day E15.5 also continued to be positive for BrdU incorporation in the Tg lenses (Fig. 5A and Fig.5B, c). This difference between Tg and WT lenses indicates that in the Tg lenses, the central epithelial cells which normally exhibit reduced proliferation at E15.5 and stay quiescent in postnatal lenses, appear to continue to proliferate, with lens fiber cells also exhibiting abnormalities of cell cycle progression. Whether these alterations are partly responsible for the increased lens size in the Tg embryonic eyes is a speculation at present. Our observation of increased lens size and increased lens epithelial cell proliferation in response to RhoGDIα overexpression is somewhat contradictory to the response reported for cardiac tissue growth (Wei et al., 2002). In the RhoGDIα overexpressed mouse heart, cell proliferation has been shown to be reduced significantly in association with increased expression of p21 (Waf1/Cip1) cell cycle inhibitor (Wei et al., 2002). However, inhibition of Rho GTPases activity has also been shown to promote progenitor cell proliferation (Ghiaur et al., 2006) indicating that the effect of Rho GTPases inhibition could be cell type specific, in the context of cell proliferation responses. In contrast to the embryonic lenses, the size of the eye and lens was much smaller (50%) compared to the wild type in the postnatal Tg mice (P1 and one month-old mice, see Fig. 1E; a and b). Although ocular tissues other than the lens showed no structural or histological abnormalities, reduced eye size in postnatal mice might be the result of a degenerated lens in the Tg animals due to increased apoptosis of lens fibers as shown in Fig. 7. Further, lens secreted factors have been shown to be critical for the development of anterior chamber and the growth of the eye (Chow and Lang, 2001; Jean et al., 1998), thus the disrupted lens in the RhoGDIα Tg mice might be also partly responsible for decreased eye growth. Similar observations have been reported in mice with disrupted lens TGF-β signaling or with homozygous deletion of c-abl interacting protein Abi-2 (de Iongh et al., 2001; Grove et al., 2004). In situ hybridization analysis at E17.5 revealed that transgene expression predominantly localized to the lens fibers (and was minimally detectable in lens epithelium), suggesting that the lens epithelial changes observed in the Tg lenses might not be solely due to inactivation of Rho GTPases in the lens epithelium. However, the transgenic mice that were developed using the αA-crystallin promoter alone did not exhibit any obvious lens defects, either at P1 or in one month-old animals (data not shown), indicating the importance of chimeric promoter for the observed phenotype induced by the RhoGDIα overexpression.
The consistent phenotype caused by RhoGDIα overexpression in the mouse lens is defective fiber cell migration, organization and attenuated fiber cell elongation. Histological analysis of E14.5 and P1 RhoGDIα Tg lenses revealed abnormal orientation of migrating fibers at the equatorial region, with secondary fibers failing to reach the epithelium and revealing blunted and globulized anterior fiber tips. These abnormalities could be the result of the observed defects in actin cytoskeletal organization and cell-cell junctions, processes regulated by Rho, Rac and Cdc42, in the migrating and elongating lens fibers (Fig. 8). Staining for filamentous actin and β-catenin in the RhoGDIβ Tg lenses showed not only much reduced staining in the fibers cells, but also abnormal distribution of actin filament, indicating disruption in actin cytoskeletal organization and cell-cell adhesions. Additionally, Tg lenses revealed abnormal fiber cell morphology and did not posses characteristic hexagonal and symmetrical fiber cell organization seen in WT lenses (Fig. 8B). Cell migration is critically dependent on Rho GTPases regulated actin cytoskeletal dynamics, formation of lamellipodia and integrin-ECM adhesions formation, and actomyosin-based cellular contraction (Ridley et al., 2003). In our earlier study we demonstrated the involvement of Rho GTPases in growth factor-induced lens epithelial actin cytoskeletal organization, focal adhesion and adherens junction formation (Maddala et al., 2003). Differentiation of lens fibers has also been shown to be associated with increases in myosin light chain phosphorylation (Maddala, 2007). Further, defective growth factor signaling has been reported to lead to defective lens fiber cell migration (de Iongh et al., 2001) and Rac GTPase has recently been suggested to be involved in cortical actin reorganization during lens fiber differentiation (Weber and Menko, 2006b). Moreover, the phenotype of RhoGDIα Tg mouse lens fiber cell migration pattern and organization was found to be somewhat similar to that of the Abi-2 null mouse lens (Grove et al., 2004). The c-abl kinase interacting proteins Abi-1 and Abi-2 regulate actin polymerization at the leading edges of migrating cells in a Rac GTPase-dependent manner by regulating WAVE /Arp 2/3 complex activity (Soderling and Scott, 2006; Stradal et al., 2001). The Abi-2 null lens phenotype has been attributed primarily to defective fiber cell migration (Grove et al., 2004). The observed similarity in lens phenotype between the RhoGDIα Tg and Abi-2 null mice further support the importance of regulated actin cytoskeletal organization and fiber cell migration for lens morphogenesis and growth. We speculate that the disrupted fiber cell migration observed in the RhoGDIα Tg mice could be primarily due to defective Rac and Cdc42 GTPase signaling activity, because in our previous study using a C3-exoenzyme-mediated selective inactivation of RhoA and RhoB GTPases, we did not note such obvious changes in the fiber cell migration patterns (Maddala et al., 2004). Conditional gene ablation studies would be required to acquire a better understanding of the specific role and involvement of Rac and Cdc42 GTPases in lens fiber cell migration and organization.
Intriguingly, the RhoGDI transgenic lenses also showed increased levels of Rho, Rac and Cdc42 Rho GTPases protein levels perhaps through negative feed back response to the inhibition of Rho GTPases by overexpression of Rho GDI. A similar observation has been reported in the transgenic heart tissue overexpressing RhoGDI (Wei et al., 2002), indicating the role of RhoGDI in the regulation of Rho GTPases expression. Interestingly though, the increased levels of Rho GTPases observed in the RhoGDI Tg lenses did not result in net increases in either the amount of activated Rho GTPase as evaluated by membrane localization (Fig. 2A and B), or in the amounts of RhoGDI unbound Rho GTPases as tested by GST-RhoGDI -Rac interaction assays (Supplemental Figure). These observations collectively support the contention that the majority of the lens changes caused by the transgenic overexpression of RhoGDI could be attributed to the inactivation of Rho GTPases.
Interestingly in the RhoGDIα Tg lenses, the levels of Ser-59 phosphorylated αB-crystallin, which are known to be regulated by p38 MAPK (Maddala and Rao, 2005), were markedly increased especially in the lens epithelium. αB-crystallin, a small heat shock protein has been shown to localize to the focal adhesions, lamellipodium, and interact with actin cytoskeleton and colocalize with Rac-regulated WAVE and Arp2/3 complexes in the migrating lens epithelial and in other cell types, in a phosphorylation-dependent manner (Launay et al., 2006; Maddala and Rao, 2005). Therefore, the increased levels of phospho-αB crystallin in RhoGDIα Tg lens epithelial cells are likely a consequence of defective interactions and cell adhesions between epithelial and fiber cells in Tg lenses. It remains unclear whether Rho GTPases directly regulate αB-crystallin phosphorylation, or whether the noted increase in αB-crystallin phosphorylation is secondary to defective lens integrity.
Rho GDIα Tg lenses showed marked decreases in the protein levels of aquaporin-0 and phakanin (Fig. 6). Additionally, the distribution of aquaporin-0 and connexin-50 in RhoGDIα expressing Tg lenses was found to be abnormal (Fig. 6). Mutations in aquaporin-0, connexin 50 and beaded filaments (phakanin and filensin) result in cataracts in mice and humans indicating a fundamental role for these proteins in lens function (Francis et al., 1999; Graw, 2004; Perng and Quinlan, 2005; Rong et al., 2002). In the RhoGDIα Tg lenses, the disrupted actin cytoskeletal organization and fiber cell elongation leading to defective fiber cell membrane organization and trafficking, might be a reasonable explanation for the disrupted organization of transmembrane aquaporin-0 and connexin-50. We have noted similar changes in RhoA and RhoB inactivated lenses in earlier studies (Maddala et al., 2004). Since the decreased levels of aquapoprin-0 and connexin-50 proteins appeared not to be related to altered gene transcription (based on cDNA microarray analysis, data not shown), we speculate that the defective cortical cytoskeletal organization in fiber cells from lenses overexpressing RhoGDI, affects the membrane trafficking of aquaporin-0 and connexin-50, perhaps leading to degradation of these proteins. Moreover, the ERM (ezrin-radixin-moesin) proteins which link the cortical actin cytoskeleton to integral membrane proteins, and control cell shape, membrane trafficking and cortical cytoskeletal organization, require Rho GTPases activity for their activation (Bretscher et al., 2002). In preliminary analyses, we noted that ERM protein phosphorylation, which is critical for the actin binding ability of these proteins, is decreased in RhoGDIα transgenic lenses (Rao and Maddala unpublished data).
In summary, this work demonstrates that the activity of Rho family of GTPases including Rho, Rac and Cdc42 is essential for lens growth and development, and that this family of small GTPases play a key role in regulating lens epithelial cell elongation, migration, membrane integrity and differentiation. Further studies focused on lens-specific and molecular targeted approaches are warranted to delineate the precise role(s) of each of the Rho family of GTPases in regulation of lens epithelial cell proliferation, elongation, migration and differentiation.
Supplementary Material
To determine and confirm the (i) interaction of lens Rho GTPases with transgenically overexpressed RhoGDI, and (ii) the subsequent inactivation of RhoGTPases via RhoGDI-Rho GTPase complex formation, an in vitro RhoGDI-Rac interaction assay was performed using recombinant GST-RhoGDI fusion protein as an affinity-based pull down reagent, as described by (Peterson et al., 2006). The GST-RhoGDI fusion protein was incubated with lens lysates of WT and Tg mice (500 μg protein, 800xg supernatant) for one hour at 4 °C. GST-sepharose was added and the mixture was rotated at 4 °C for 30 minutes. Following this, the sepharose beads were pelleted and washed extensively. The GST-sepharose bound proteins were solubulized in Laemmli protein sample buffer and separated on the SDS-polyacrylamide gel (12.5% acrylamide), then transferred to nitrocellulose membranes and immunoblotted with anti-Rac1 GTPase antibody. The results of this experiment revealed that while the lens lysates derived from the WT mice show an easily detectable specific immunopositive band for the Rac1 GTPase in the GST-RhoGDI pulled-down protein complexes, the transgenic lens lysates showed a complete absence of Rac GTPase immunopositive protein in the GST-RhoGDI pulled-down complexes. This data confirm that the transgenically expressed RhoGDI engages a predominant proportion of the Rac GTPase in these lenses, such that there exists little to no free Rac GTPase to interact with the recombinant GST-RhoGDI in transgenic lenses as compared to the WT lenses. Since Rho GDIα has been shown to have similar binding and inhibitory activity towards all three Rho GTPases including Rho, Rac and Cdc42 (Olofsson, 1999), we speculate that both Rho and Cdc42 like in the case of Rac1, are inactivated in the Tg lenses by forming the complexes with transgenically expressed RhoGDIα.
Acknowledgements
We thank Drs. Sam Zigler for anti-gamma crystallin, anti-betaB2 crystallin and aquaporin-0 antibodies, Roy Quinlan for anti-CP-49 antibody, Joe Horwitz for anti-alpha crystallin antibodies, Yoshimi Takai for bovine RhoGDIα cDNA plasmid and Patrick Casey for the recombinant GST-RhoGDI protein. We thank Jianming Qiu for technical assistance and Drs. Padmini Rao and Shelia Baker for a critical reading of the manuscript. This work was supported by NIH R01 grants EY12201, EY013573 (PVR), EY13146 (LWR) and P30EY005722 and EY14795 (Core grants) and Research to Prevent Blindness (PVR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adra CN, Manor D, Ko JL, Zhu S, Horiuchi T, Van Aelst L, Cerione RA, Lim B. RhoGDIgamma: a GDP-dissociation inhibitor for Rho proteins with preferential expression in brain and pancreas. Proc Natl Acad Sci U S A. 1997;94:4279–84. doi: 10.1073/pnas.94.9.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassnett S, Missey H, Vucemilo I. Molecular architecture of the lens fiber cell basal membrane complex. J Cell Sci. 1999;112(Pt 13):2155–65. doi: 10.1242/jcs.112.13.2155. [DOI] [PubMed] [Google Scholar]
- Beebe DC, Vasiliev O, Guo J, Shui YB, Bassnett S. Changes in adhesion complexes define stages in the differentiation of lens fiber cells. Invest Ophthalmol Vis Sci. 2001;42:727–34. [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol. 2002;3:586–99. doi: 10.1038/nrm882. [DOI] [PubMed] [Google Scholar]
- Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–79. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- Cernuda-Morollon E, Ridley AJ. Rho GTPases and leukocyte adhesion receptor expression and function in endothelial cells. Circ Res. 2006;98:757–67. doi: 10.1161/01.RES.0000210579.35304.d3. [DOI] [PubMed] [Google Scholar]
- Chen Y, Stump RJ, Lovicu FJ, McAvoy JW. A role for Wnt/planar cell polarity signaling during lens fiber cell differentiation? Semin Cell Dev Biol. 2006;17:712–25. doi: 10.1016/j.semcdb.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow RL, Lang RA. Early eye development in vertebrates. Annu Rev Cell Dev Biol. 2001;17:255–96. doi: 10.1146/annurev.cellbio.17.1.255. [DOI] [PubMed] [Google Scholar]
- Courtois Y, Arruti C, Barritault D, Tassin J, Olivie M, Hughes RC. Modulation of the shape of epithelial lens cells in vitro directed by a retinal extract factor. A model of interconversions and the role of actin filaments and fibronectin. Differentiation. 1981;18:11–27. doi: 10.1111/j.1432-0436.1981.tb01100.x. [DOI] [PubMed] [Google Scholar]
- de Iongh RU, Lovicu FJ, Overbeek PA, Schneider MD, Joya J, Hardeman ED, McAvoy JW. Requirement for TGFbeta receptor signaling during terminal lens fiber differentiation. Development. 2001;128:3995–4010. doi: 10.1242/dev.128.20.3995. [DOI] [PubMed] [Google Scholar]
- DerMardirossian C, Bokoch GM. GDIs: central regulatory molecules in Rho GTPase activation. Trends Cell Biol. 2005;15:356–63. doi: 10.1016/j.tcb.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Dovas A, Couchman JR. RhoGDI: multiple functions in the regulation of Rho family GTPase activities. Biochem J. 2005;390:1–9. doi: 10.1042/BJ20050104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–35. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- Francis PJ, Berry V, Moore AT, Bhattacharya S. Lens biology: development and human cataractogenesis. Trends Genet. 1999;15:191–6. doi: 10.1016/s0168-9525(99)01738-2. [DOI] [PubMed] [Google Scholar]
- Fukumoto Y, Kaibuchi K, Hori Y, Fujioka H, Araki S, Ueda T, Kikuchi A, Takai Y. Molecular cloning and characterization of a novel type of regulatory protein (GDI) for the rho proteins, ras p21-like small GTP-binding proteins. Oncogene. 1990;5:1321–8. [PubMed] [Google Scholar]
- Ghiaur G, Lee A, Bailey J, Cancelas JA, Zheng Y, Williams DA. Inhibition of RhoA GTPase activity enhances hematopoietic stem and progenitor cell proliferation and engraftment. Blood. 2006;108:2087–94. doi: 10.1182/blood-2006-02-001560. [DOI] [PubMed] [Google Scholar]
- Graw J. Congenital hereditary cataracts. Int J Dev Biol. 2004;48:1031–44. doi: 10.1387/ijdb.041854jg. [DOI] [PubMed] [Google Scholar]
- Grove M, Demyanenko G, Echarri A, Zipfel PA, Quiroz ME, Rodriguiz RM, Playford M, Martensen SA, Robinson MR, Wetsel WC, Maness PF, Pendergast AM. ABI2-deficient mice exhibit defective cell migration, aberrant dendritic spine morphogenesis, and deficits in learning and memory. Mol Cell Biol. 2004;24:10905–22. doi: 10.1128/MCB.24.24.10905-10922.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the control of cell behaviour. Biochem Soc Trans. 2005;33:891–5. doi: 10.1042/BST20050891. [DOI] [PubMed] [Google Scholar]
- Horwitz J. Alpha-crystallin. Exp Eye Res. 2003;76:145–53. doi: 10.1016/s0014-4835(02)00278-6. [DOI] [PubMed] [Google Scholar]
- Jean D, Ewan K, Gruss P. Molecular regulators involved in vertebrate eye development. Mech Dev. 1998;76:3–18. doi: 10.1016/s0925-4773(98)00117-8. [DOI] [PubMed] [Google Scholar]
- Launay N, Goudeau B, Kato K, Vicart P, Lilienbaum A. Cell signaling pathways to alphaB-crystallin following stresses of the cytoskeleton. Exp Cell Res. 2006;312:3570–84. doi: 10.1016/j.yexcr.2006.07.025. [DOI] [PubMed] [Google Scholar]
- Lee A, Fischer RS, Fowler VM. Stabilization and remodeling of the membrane skeleton during lens fiber cell differentiation and maturation. Dev Dyn. 2000;217:257–70. doi: 10.1002/(SICI)1097-0177(200003)217:3<257::AID-DVDY4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Lelias JM, Adra CN, Wulf GM, Guillemot JC, Khagad M, Caput D, Lim B. cDNA cloning of a human mRNA preferentially expressed in hematopoietic cells and with homology to a GDP-dissociation inhibitor for the rho GTP-binding proteins. Proc Natl Acad Sci U S A. 1993;90:1479–83. doi: 10.1073/pnas.90.4.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo WK, Shaw AP, Wen XJ. Actin filament bundles in cortical fiber cells of the rat lens. Exp Eye Res. 1997;65:691–701. doi: 10.1006/exer.1997.0375. [DOI] [PubMed] [Google Scholar]
- Lovicu FJ, McAvoy JW. Growth factor regulation of lens development. Dev Biol. 2005;280:1–14. doi: 10.1016/j.ydbio.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Maddala R, Deng PF, Costello JM, Wawrousek EF, Zigler JS, Rao VP. Impaired cytoskeletal organization and membrane integrity in lens fibers of a Rho GTPase functional knockout transgenic mouse. Lab Invest. 2004;84:679–92. doi: 10.1038/labinvest.3700105. [DOI] [PubMed] [Google Scholar]
- Maddala R, Peng YW, Rao PV. Selective expression of the small GTPase RhoB in the early developing mouse lens. Dev Dyn. 2001;222:534–7. doi: 10.1002/dvdy.1202. [DOI] [PubMed] [Google Scholar]
- Maddala R, Rao VP. alpha-Crystallin localizes to the leading edges of migrating lens epithelial cells. Exp Cell Res. 2005;306:203–15. doi: 10.1016/j.yexcr.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Maddala R, Reddy VN, Epstein DL, Rao V. Growth factor induced activation of Rho and Rac GTPases and actin cytoskeletal reorganization in human lens epithelial cells. Mol Vis. 2003;9:329–36. [PubMed] [Google Scholar]
- Maddala R, Skiba N, Rao PV. Lens Fiber Cell Elongation and Differentiation is Associated with a Robust Increase in Myosin Light Chain Phosphorylation in the Developing Mouse. Differentiation. 2007;75:713–25. doi: 10.1111/j.1432-0436.2007.00173.x. [DOI] [PubMed] [Google Scholar]
- Mousa GY, Trevithick JR. Differentiation of rat lens epithelial cells in tissue culture. II. Effects of cytochalasins B and D on actin organization and differentiation. Dev Biol. 1977;60:14–25. doi: 10.1016/0012-1606(77)90107-5. [DOI] [PubMed] [Google Scholar]
- Olofsson B. Rho guanine dissociation inhibitors: pivotal molecules in cellular signalling. Cell Signal. 1999;11:545–54. doi: 10.1016/s0898-6568(98)00063-1. [DOI] [PubMed] [Google Scholar]
- Perng MD, Quinlan RA. Seeing is believing! The optical properties of the eye lens are dependent upon a functional intermediate filament cytoskeleton. Exp Cell Res. 2005;305:1–9. doi: 10.1016/j.yexcr.2004.11.021. [DOI] [PubMed] [Google Scholar]
- Peterson YK, Kelly P, Weinbaum CA, Casey PJ. A novel protein geranylgeranyltransferase-I inhibitor with high potency, selectivity, and cellular activity. J Biol Chem. 2006;281:12445–50. doi: 10.1074/jbc.M600168200. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J. Lens differentiation in vertebrates. A review of cellular and molecular features. Differentiation. 1981;19:134–53. doi: 10.1111/j.1432-0436.1981.tb01141.x. [DOI] [PubMed] [Google Scholar]
- Ramaekers F, Jap P, Mungyer G, Bloemendal H. Microfilament assembly during lens cell elongation in vitro. Curr Eye Res. 1982;2:169–81. doi: 10.3109/02713688208997691. [DOI] [PubMed] [Google Scholar]
- Ramaekers FC, Boomkens TR, Bloemendal H. Cytoskeletal and contractile structures in bovine lens cell differentiation. Exp Cell Res. 1981;135:454–61. doi: 10.1016/0014-4827(81)90190-7. [DOI] [PubMed] [Google Scholar]
- Rao PV, Maddala R. The role of the lens actin cytoskeleton in fiber cell elongation and differentiation. Semin Cell Dev Biol. 2006;17:698–711. doi: 10.1016/j.semcdb.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reneker LW, Chen Q, Bloch A, Xie L, Schuster G, Overbeek PA. Chick delta1-crystallin enhancer influences mouse alphaA-crystallin promoter activity in transgenic mice. Invest Ophthalmol Vis Sci. 2004;45:4083–90. doi: 10.1167/iovs.03-1270. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science. 2003;302:1704–9. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- Rong P, Wang X, Niesman I, Wu Y, Benedetti LE, Dunia I, Levy E, Gong X. Disruption of Gja8 (alpha8 connexin) in mice leads to microphthalmia associated with retardation of lens growth and lens fiber maturation. Development. 2002;129:167–74. doi: 10.1242/dev.129.1.167. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Takai Y. The Rho small G protein family-Rho GDI system as a temporal and spatial determinant for cytoskeletal control. Biochem Biophys Res Commun. 1998;245:641–5. doi: 10.1006/bbrc.1998.8253. [DOI] [PubMed] [Google Scholar]
- Scherle P, Behrens T, Staudt LM. Ly-GDI, a GDP-dissociation inhibitor of the RhoA GTP-binding protein, is expressed preferentially in lymphocytes. Proc Natl Acad Sci U S A. 1993;90:7568–72. doi: 10.1073/pnas.90.16.7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderling SH, Scott JD. WAVE signalling: from biochemistry to biology. Biochem Soc Trans. 2006;34:73–6. doi: 10.1042/BST0340073. [DOI] [PubMed] [Google Scholar]
- Stradal T, Courtney KD, Rottner K, Hahne P, Small JV, Pendergast AM. The Abl interactor proteins localize to sites of actin polymerization at the tips of lamellipodia and filopodia. Curr Biol. 2001;11:891–5. doi: 10.1016/s0960-9822(01)00239-1. [DOI] [PubMed] [Google Scholar]
- Sue Menko A. Lens epithelial cell differentiation. Exp Eye Res. 2002;75:485–90. doi: 10.1006/exer.2002.2057. [DOI] [PubMed] [Google Scholar]
- Togawa A, Miyoshi J, Ishizaki H, Tanaka M, Takakura A, Nishioka H, Yoshida H, Doi T, Mizoguchi A, Matsuura N, Niho Y, Nishimune Y, Nishikawa S, Takai Y. Progressive impairment of kidneys and reproductive organs in mice lacking Rho GDIalpha. Oncogene. 1999;18:5373–80. doi: 10.1038/sj.onc.1202921. [DOI] [PubMed] [Google Scholar]
- Ueda T, Kikuchi A, Ohga N, Yamamoto J, Takai Y. Purification and characterization from bovine brain cytosol of a novel regulatory protein inhibiting the dissociation of GDP from and the subsequent binding of GTP to rhoB p20, a ras p21-like GTP-binding protein. J Biol Chem. 1990;265:9373–80. [PubMed] [Google Scholar]
- Van Aelst L, D’Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- Weber GF, Menko AS. Actin filament organization regulates the induction of lens cell differentiation and survival. Dev Biol. 2006a;295:714–29. doi: 10.1016/j.ydbio.2006.03.056. [DOI] [PubMed] [Google Scholar]
- Weber GF, Menko AS. Phosphatidylinositol 3-kinase is necessary for lens fiber cell differentiation and survival. Invest Ophthalmol Vis Sci. 2006b;47:4490–9. doi: 10.1167/iovs.06-0401. [DOI] [PubMed] [Google Scholar]
- Wei L, Imanaka-Yoshida K, Wang L, Zhan S, Schneider MD, DeMayo FJ, Schwartz RJ. Inhibition of Rho family GTPases by Rho GDP dissociation inhibitor disrupts cardiac morphogenesis and inhibits cardiomyocyte proliferation. Development. 2002;129:1705–14. doi: 10.1242/dev.129.7.1705. [DOI] [PubMed] [Google Scholar]
- Xie L, Overbeek PA, Reneker LW. Ras signaling is essential for lens cell proliferation and lens growth during development. Dev Biol. 2006;298:403–14. doi: 10.1016/j.ydbio.2006.06.045. [DOI] [PubMed] [Google Scholar]
- Zelenka PS. Regulation of cell adhesion and migration in lens development. Int J Dev Biol. 2004;48:857–65. doi: 10.1387/ijdb.041871pz. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
To determine and confirm the (i) interaction of lens Rho GTPases with transgenically overexpressed RhoGDI, and (ii) the subsequent inactivation of RhoGTPases via RhoGDI-Rho GTPase complex formation, an in vitro RhoGDI-Rac interaction assay was performed using recombinant GST-RhoGDI fusion protein as an affinity-based pull down reagent, as described by (Peterson et al., 2006). The GST-RhoGDI fusion protein was incubated with lens lysates of WT and Tg mice (500 μg protein, 800xg supernatant) for one hour at 4 °C. GST-sepharose was added and the mixture was rotated at 4 °C for 30 minutes. Following this, the sepharose beads were pelleted and washed extensively. The GST-sepharose bound proteins were solubulized in Laemmli protein sample buffer and separated on the SDS-polyacrylamide gel (12.5% acrylamide), then transferred to nitrocellulose membranes and immunoblotted with anti-Rac1 GTPase antibody. The results of this experiment revealed that while the lens lysates derived from the WT mice show an easily detectable specific immunopositive band for the Rac1 GTPase in the GST-RhoGDI pulled-down protein complexes, the transgenic lens lysates showed a complete absence of Rac GTPase immunopositive protein in the GST-RhoGDI pulled-down complexes. This data confirm that the transgenically expressed RhoGDI engages a predominant proportion of the Rac GTPase in these lenses, such that there exists little to no free Rac GTPase to interact with the recombinant GST-RhoGDI in transgenic lenses as compared to the WT lenses. Since Rho GDIα has been shown to have similar binding and inhibitory activity towards all three Rho GTPases including Rho, Rac and Cdc42 (Olofsson, 1999), we speculate that both Rho and Cdc42 like in the case of Rac1, are inactivated in the Tg lenses by forming the complexes with transgenically expressed RhoGDIα.