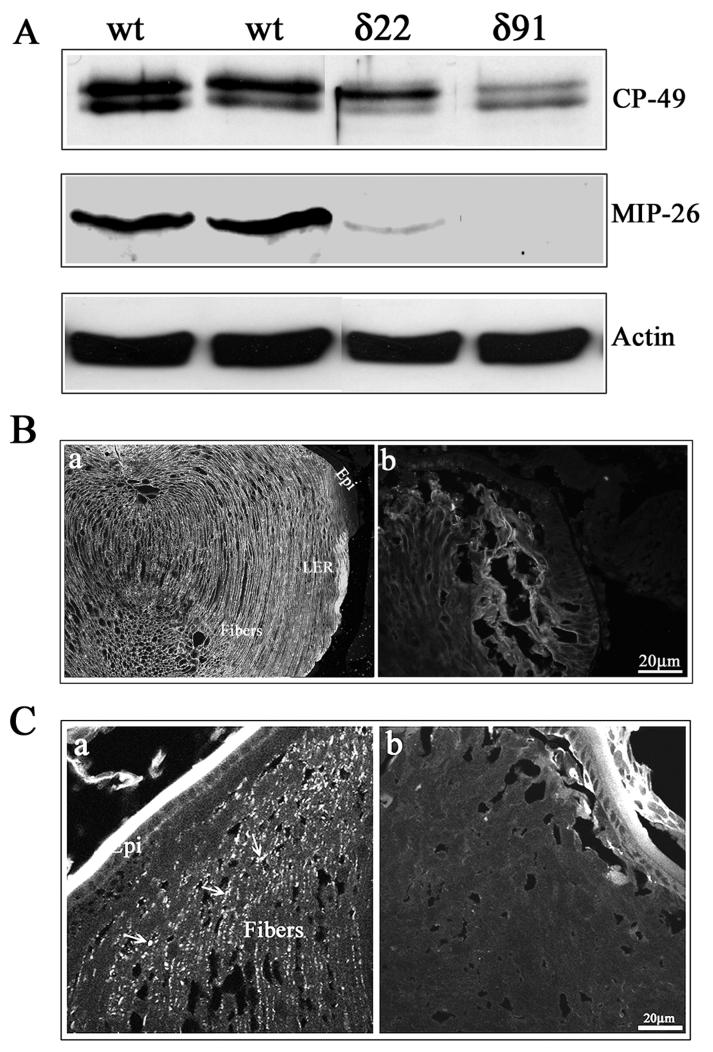
Fig. 6.
Defective organization of water channel and gap junctional proteins in the RhoGDIα transgenic lens fibers. A. P1 Tg lens homogenates (800xg supernatants) showed decreased levels of fiber-specific beaded filament protein (CP-49, also called phakanin), water channel protein Aquaporin-0 (also called MIP-26) compared to WT lenses, as determined by western blot analysis. Actin, which was immunoblotted to confirm equality of protein loading, indicated no differences between the WT and Tg specimens. B. Immunofluorescence distribution of aquaporin-0 in P1 WT and Tg lens cryosections. While immunolabelling for aquaporin-0 shows a fiber cell specific distribution along the cell membrane in WT lenses, a much weaker immunostaining that was not specifically localized to the lens fiber cell membrane, was noted in the case of Tg lenses. C. Immunofluorescence distribution of connexin-50, a lens fiber-specific gap junction protein in Tg and WT lenses. Similar to aquaporin-0, WT lenses reveal a specific and punctuate immunopositive staining pattern for connexin-50 distributed along the fiber cell membrane (panel a, indicated with arrows), while there was no detectable immunopositive labeling for connexin-50 in the Tg lenses (b). The lens capsule exhibits a non-specific bright staining in both WT and Tg specimens.