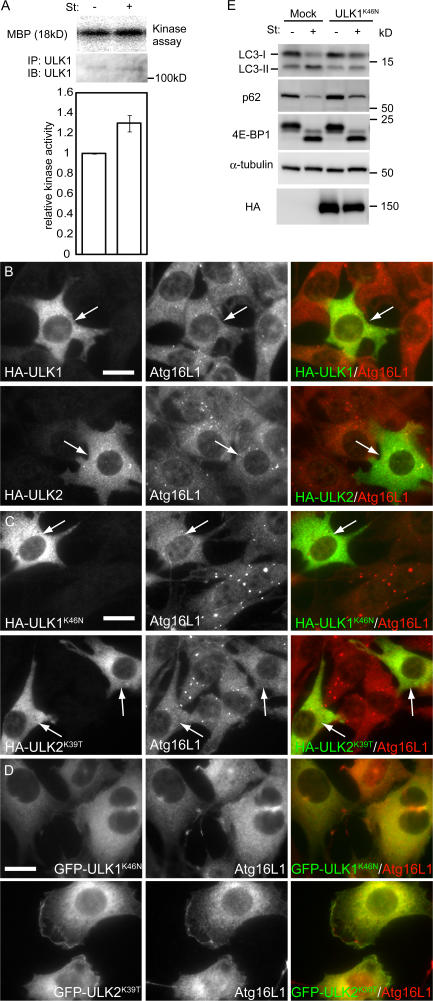
Figure 2.
The kinase-dead ULK mutants inhibit autophagy. (A) In vitro kinase assay of endogenous ULK1. MEFs were cultured in complete or starvation medium for 60 min. ULK1 kinase activity was determined as described in Materials and methods. Relative kinase activity is shown. Data are the mean ± SE of five independent experiments. (B and C) NIH3T3 cells were transiently transfected with HA-ULK1, HA-ULK2, or their kinase-dead mutants and subjected to immunofluoresence microscopy using monoclonal anti-HA and polyclonal anti-Atg16L1 antibodies for primary staining and Alexa Fluor 488–conjugated goat anti–mouse IgG and Alexa Fluor 568–conjugated goat anti–rabbit IgG antibodies for secondary antibodies. Transfected cells are indicated by arrows. Bars, 20 μm. (D) NIH3T3 cells stably expressing GFP-ULK1K46N and GFP-ULK2K39T were cultured in starvation medium for 120 min. The cells were subjected to immunofluorescence microscopy using anti-Atg16L1 antibody. Bar, 20 μm. (E) NIH 3T3 cells were transfected with the retroviral vectors encoding HA-ULK1K46N or with the corresponding empty retrovirus (mock). They were cultured in complete or starvation medium for 120 min. Cell lysates were then analyzed by immunoblot analysis with the indicated antibodies.