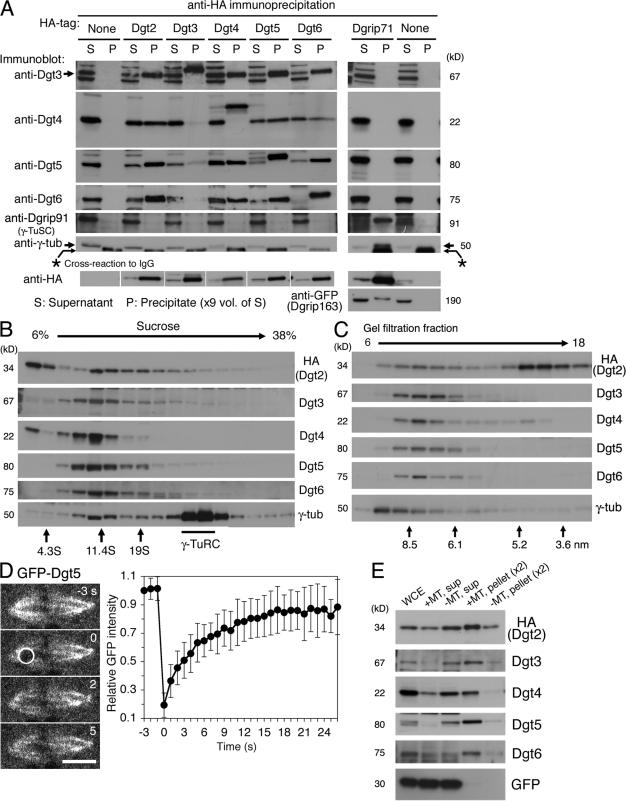
Figure 1.
Dgt2–6 proteins form a complex and associate with MTs. (A) Dgt2–6 proteins coimmunoprecipitate with one another but not with γ-TuRC subunits. Supernatant (S) and precipitated (P) fractions after extract incubation with anti-HA beads were immunoblotted for specific subunits. White lines indicate that intervening lanes have been spliced out. (B and C) Sucrose gradient sedimentation (B) and gel filtration chromatography (C) of Dgt2–6 proteins and γ-tubulin. Dgt proteins had a common peak at 11S and 7.5 nm, whereas the majority of γ-tubulin was in the larger γ-TuRC complex. The positions of standard markers are indicated. (D) FRAP analysis of GFP-Dgt5 on the spindle (n = 17). Bleached area is indicated by a white circle. Bar, 5 μm. Error bars show SD. See also Video 1 (available at http://www.jcb.org/cgi/content/full/jcb.200711053/DC1). (E) MT cosedimentation assay. Significant portions of Dgt2–6 proteins, but not control GFP, cosedimentated with MTs (pellet). WCE; whole cell extract.