Figure 4.
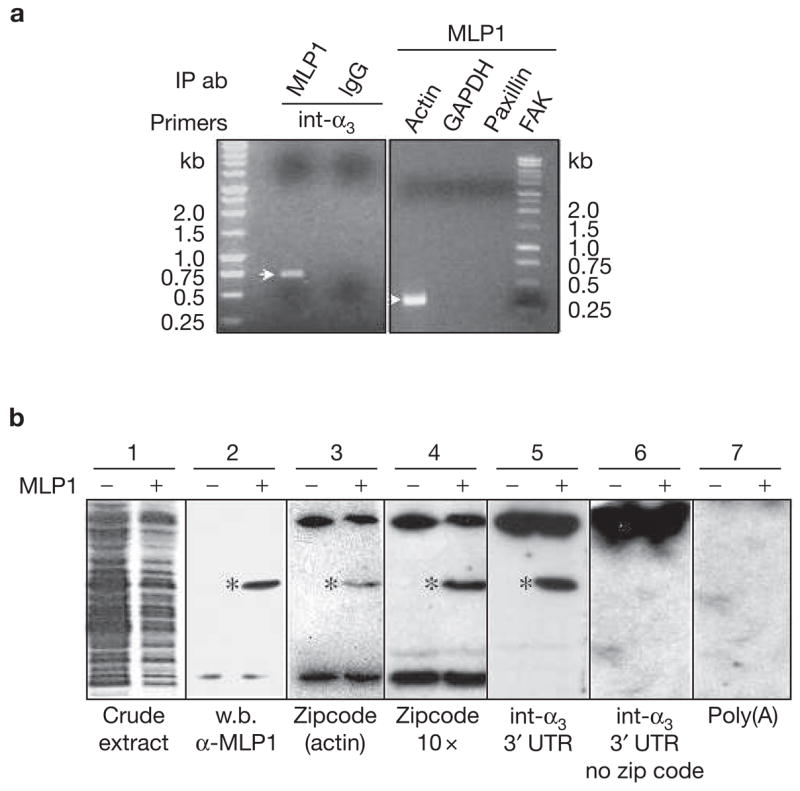
MLP1 interacts with integrin α3 3′ untranslated region (UTR) in vivo and in vitro. (a) H520 cells were transfected with 10× m.o.i. of adenovirus-containing MLP1 coding sequence and total RNAs were subjected to RNA pulldown and reverse transcription polymerase chain reaction (RT-PCR). Integrin α3 (int-α3) RNA was detected in the fraction that was pulled down by MLP1 antibody, yielding an expected fragment size (see Methods) on an ethidium-bromide-stained agarose gel. Pulldown by purified rabbit immunoglobulin G (IgG) served as a negative control. In addition, actin mRNA was present in the MLP1-containing complex, which was detected by RT-PCR yielding the expected 337-bp fragment, whereas paxillin, focal adhesion protein (FAK) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were not detectable. IP ab, immunoprecipitated antibodies (b) Extracts from BL21<DE3> bacteria transformed with (+) or without (−) plasmids containing MLP1 were run on SDS–PAGE and stained with Coomassie brilliant blue (blot 1) or western-blotted (w.b.) with anti-MLP1 antibody (blot 2). The MLP1 protein band is marked (*). Duplicate lanes were transferred to nitrocellulose membrane, renatured and hybridized with various 32P-labelled RNAs indicated at the bottom (blots 3–7). MLP1 (*) binds only to RNA molecules containing the zipcode (blots 3, 4 and 5). The bands at the top and bottom of the gels, present in both MLP1+ and MLP1− samples, were probably bacterial RNA-binding proteins.
