Abstract
Recent advances in transplantation, oncology and AIDS therapy have greatly increased life expectancies of patients diagnosed with malignancy, auto-immune disorders and organ failure. However, as this immune compromised population grows, complications of such therapies have become a major source of morbidity and mortality. Classical clinical and laboratory evidence of intra-abdominal pathology may be absent in the immune compromised host. Consequently, the radiologist is increasingly called upon to diagnose acute intra-abdominal complications associated with immunodeficiency. This review explores the aetiology of the acute abdomen in the immune compromised host. The typical radiological appearances of the commonest conditions are illustrated. The challenges and limitations in the radiological diagnosis of these conditions are discussed.
Keywords: Immunocompromised, acute abdomen
Introduction
Modern advances in immunosuppressive therapies and chemotherapy have greatly increased the life expectancy of patients who would previously have succumbed to malignancy, auto-immune disease or organ failure. However, these therapies may cause significant morbidity and mortality in their own right. As the immune compromised population grows, physicians and radiologists must become increasingly familiar with the diverse and unusual complications of treatment encountered in these patients.
Immune compromised patients are a heterogeneous group including those receiving chemotherapy for malignant disease, those receiving steroids for auto-immune disease, post-transplant patients and those with AIDS (Table 1). Possible causes of an acute abdomen in the immune compromised host include opportunistic infections, iatrogenic pathology or complications specific to the underlying primary disease (Table 2).
Table 1.
Classification of immune compromised patients.
| Mild-to-moderate | Elderly |
| immunocompromise | Malnourished |
| Diabetes | |
| Uraemia | |
| Steroid medication | |
| Malignancy (not on chemotherapy) | |
| Post-transplant (maintenance immunosuppressive therapy) | |
| AIDS (CD4+ count >200/mm3) | |
| Severe | AIDS (CD4+ count <200/mm3) |
| immunocompromise | Malignancy (induction chemotherapy/neutrophil count <1000/mm3) |
| Post-transplant (high dose immunosuppressive therapy) |
Table 2.
Causes of the acute abdomen in the immune compromised patient.
| Opportunistic infections | Pseudomembranous colitis |
| Typhlitis (neutropenic colitis) | |
| Cytomegalovirus (CMV) colitis/oesophagitis/gastritis | |
| Mycobacterial enteritis | |
| Cryptosporidium gastritis | |
| AIDS-related cholangitis | |
| Hepatosplenic abscesses – pyogenic/fungal | |
| Treatment related conditions | Intestinal graft-versus-host disease (GVHD) |
| Peptic ulceration – corticosteroids/anti-metabolite drugs | |
| Pancreatitis – corticosteroids | |
| Indinavir renal stones – HIV | |
| Hepatic veno-occlusive disease – chemotherapeutic agents | |
| Exacerbation of pre-existing inflammatory bowel disease/diverticular disease post-radiotherapy | |
| Complications of primary pathology (may occur due to lymphadenopathy or AIDS-related neoplasms of the GI tract) | Bowel perforation |
| Bowel obstruction/intussusception | |
| Gastro-intestinal haemorrhage | |
| Biliary tract obstruction |
Classical clinical signs of an acute abdomen may be absent in the immune compromised patient. This paucity of symptoms, clinical signs and laboratory evidence may lead to delay in diagnosis and subsequent treatment of the underlying condition.
This article discusses the causes of the acute abdomen in the immune compromised host. Typical radiological features are illustrated using multiple imaging modalities. The potential limitations in the radiological diagnosis of these conditions are discussed.
Gastro-intestinal tract: colitis
Pseudomembranous colitis
Pseudomembranous colitis (PMC) is an acute infectious colitis caused by toxins produced by an unopposed overgrowth of Clostridium difficile bacteria in the colon. Although classically a complication of antibiotic therapy, PMC is increasingly seen in the immune compromised population with diseases such as lymphoma, leukaemia and AIDS. It is now also being seen with increasing frequency in hospitalised patients who are immune-competent.
Clinical manifestations may include diarrhoea, dehydration, abdominal pain, fever and leucocytosis. The majority of patients infected with Clostridium difficile are asymptomatic or show only mild symptoms. In severe cases, colitis may progress to toxic megacolon and colonic perforation due to full thickness colonic necrosis.
Traditionally the diagnosis of PMC depended on the demonstration of C difficile toxin on stool assays and the presence of characteristic elevated yellow mucosal plaques producing pseudomembranes on sigmoidoscopy. However, sigmoidoscopy may be negative or reveal non-specific colitis. The combination of vague clinical symptoms and signs, the non-specific sigmoidoscopy findings and stool assay results which typically take 48 h to process results in the radiologist often being the first to suggest a diagnosis of PMC.
Approximately 5% of patients with PMC present with symptoms and signs of acute abdomen or abdominal sepsis[1]. In these cases, CT can suggest the diagnosis and avoid laparotomy. Overall mortality rates from 1.1% to 3.5% have been reported for patients with PMC[2].
Plain abdominal radiographic appearances of PMC (see Table 3)
Table 3.
Plain abdominal radiographic changes in PMC[3].
| Radiographic findings | Frequency (%) |
|---|---|
| Normal radiograph | 50–68 |
| Thumbprinting | 20–50 |
| Colonic ileus | 32 |
| Small bowel ileus | 20 |
| Nodular haustral thickening | 18 |
| Ascites | 7 |
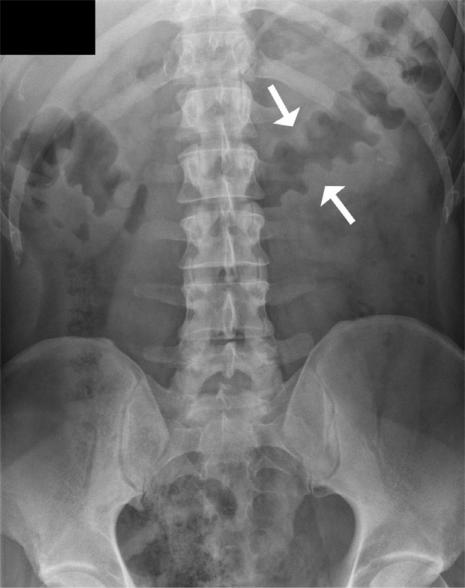
Plain abdominal radiographs may range from being normal, to showing non-specific features such as paralytic ileus and ascites through to more specific features of colonic oedema such as thumbprinting and haustral thickening (Fig. 1). In its most severe forms, complications such as toxic megacolon or perforation may be seen.
Figure 1.
Plain abdominal radiograph of a patient with PMC demonstrates haustral thickening and thumbprinting within the transverse colon (arrows).
Bowland et al.[3] found nodular haustral thickening to be the most specific feature of PMC. While more common than nodular haustral thickening, thumbprinting is less specific in the diagnosis of PMC and is also seen in ischaemic colitis, ulcerative colitis and Crohn's colitis.
Ultrasound appearance of PMC
Downey and Wilson[4] found that widespread moderate to severe colonic wall thickening (6–28 mm) in conjunction with ascites in a high risk patient cohort made a diagnosis of PMC a high probability. Ascites was found in 77% of their patients at ultrasound.
CT appearance of PMC (see Table 4)
Table 4.
Reported frequency of CT findings in pseudomembranous colitis [5–7].
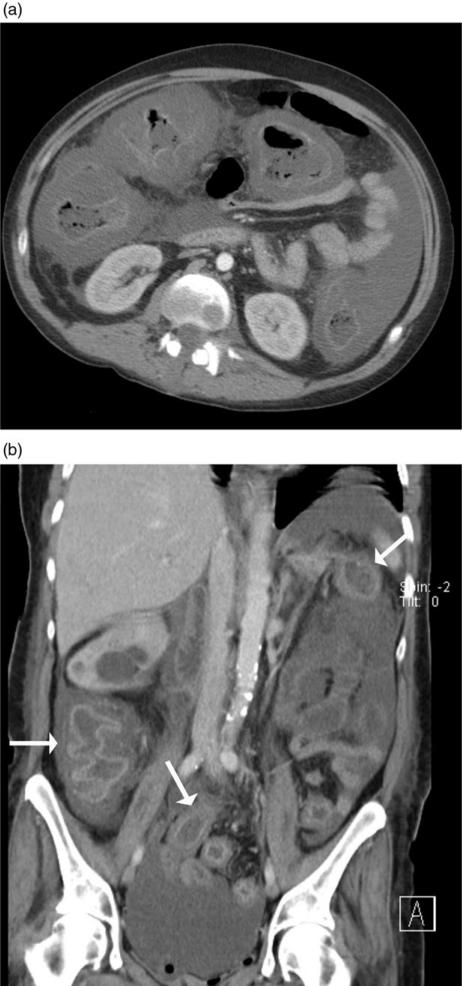
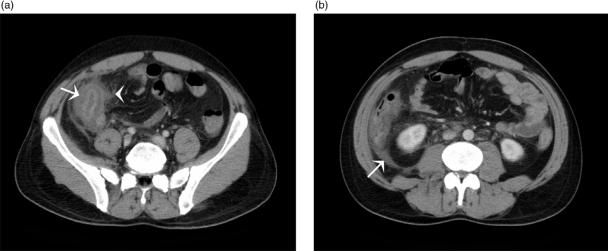
Colonic wall thickening (>4 mm) is the most common CT finding in PMC, although this finding is not specific. Ranges of wall thickening up to 32 mm have been described with means of 11–15 mm[5,6]. This degree of wall thickening is generally greater than in other forms of infectious or inflammatory forms of colitides[8,9]. Mural thickening may be circumferential or eccentric, and smooth, irregular or polypoid. The mucosa typically enhances markedly following intravenous contrast (Fig. 2).
Figure 2.
(a) Axial MDCT of a patient with PMC shows marked circumferential thickening of the ascending, transverse and descending colon with mucosal enhancement and submucosal oedema. A moderate amount of ascites is noted. (b) Coronal reformatted MDCT of a patient with PMC showing a thickened colon with associated mucosal enhancement and submucosal oedema (arrows).
Peri-colic stranding is usually mild, reflecting the primarily mucosal and submucosal nature of PMC. This finding helps differentiate PMC from other colitides. Although commonly manifesting as a pancolitis (36–47% of cases[5,6]), it may also be limited to short segments of the colon. Ascites is present in up to 40% of cases.
The ‘target sign’ or ‘double halo sign’ is also a relatively common although non-specific finding in PMC indicating mucosal hyperaemia and submucosal oedema following intravenous contrast[10] (Fig. 3). It is seen rarely in malignancy and suggests that thickened bowel is most likely caused by inflammatory disease as opposed to a neoplasm.
Figure 3.
Axial MDCT in a patient with PMC and associated toxic megacolon showing submucosal oedema and mucosal enhancement of the ascending and descending colon giving the appearance of the “target sign”. The colon is dilated; this therefore constitutes a surgical emergency.
The ‘accordion sign’ may be seen in patients who have received oral contrast. The soft-tissue component of the accordion sign represents thickening of the haustral folds due to oedema. Small amounts of oral contrast may become trapped between the thickened folds resulting in bands of alternating lower and higher attenuation that have been likened to the appearance of an accordion (Fig. 4).
Figure 4.
Axial MDCT of a patient with AIDS and PMC shows extensive nodular wall thickening throughout the transverse colon. Lines of high attenuation are from oral contrast trapped between lower attenuation nodular haustral folds resulting in the ‘accordion’ sign. A percutaneous drain is present in an abscess in segment 5 of the liver secondary to the colitis.
While the accordion sign has been previously described as a finding specific for PMC[6], it has also been recognised in ischaemic colitis and oedema secondary to portal hypertension. It is one of the least common CT findings of PMC, present in 4%[11] to 19%[12] of cases.
CT in proven cases of PMC may be normal in up to 14% of cases. Thus a negative scan does not exclude the presence of PMC.
Typhlitis
Typhlitis or neutropenic enterocolitis is a clinical syndrome in neutropenic patients characterised by fever and abdominal pain, particularly in the right iliac fossa[13]. Typically symptoms occur 10–14 days after initiation of cytotoxic chemotherapy. The condition is thought to arise due to a combination of mucosal injury by leukaemic infiltration or cytotoxic drugs, neutropenia and impaired host defences to intestinal bacteria[13].
Radiological appearances of typhlitis
Plain abdominal radiographs are non-specific in the diagnosis of typhlitis. Findings include thickened dilated loops of bowel in the right lower quadrant, thumbprinting or localised pneumatosis intestinalis. Bowel wall thickening may also be detected sonographically although this too is non-specific.
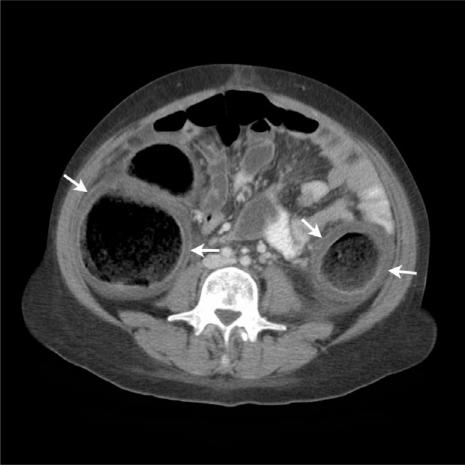
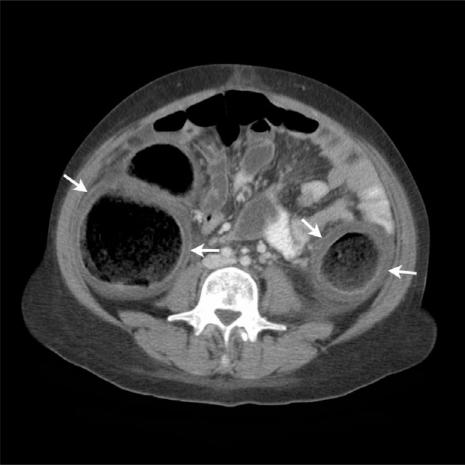
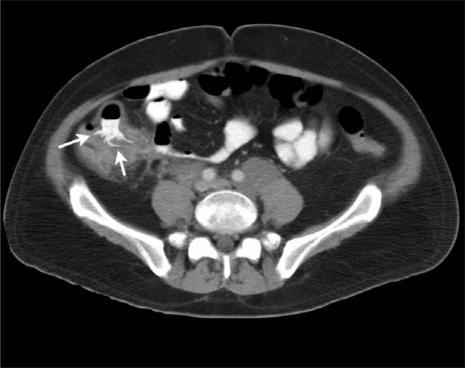
The most consistent CT finding in typhlitis is large bowel wall thickening (Fig. 5). The degree of thickening is generally less marked than that seen in PMC (mean thickness 7 mm; range 4–15 mm[14]). The degree of wall thickening significantly correlates with the outcome of patients with typhlitis. In one study, 60% of patients with wall thickening >10 mm died from this condition, compared with only 4.2% of those with mural thickness <10 mm[15].
Figure 5.
(a) Axial MDCT image of a patient presenting with typhlitis following chemotherapy. Note the caecal thickening with mucosal enhancement (arrow) and associated peri-colic mesenteric stranding (arrowhead). (b) Axial MDCT image of a neutropenic patient with typhlitis showing a thickened, enhancing ascending colon, peri-colic fluid (arrow) and mesenteric stranding. Note the relative sparing of the transverse colon and proximal small bowel loops.
The right colon is most commonly affected, up to 75% of cases in some series. Isolated caecal involvement occurs in up to 30% of cases[14]. Changes may also be seen in the remainder of the colon and in small bowel. Indeed, the combined involvement of both large and small bowel points towards a more likely diagnosis of typhlitis.
Pneumatosis intestinalis has been reported in up to 20% of cases of typhlitis. This is not seen in PMC or intestinal GVHD and is thereby more specific for typhlitis. The CT findings and discriminatory features are summarised in Tables 5 and 8.
Table 5.
CT findings in typhlitis[14].
| CT findings | Frequency (%) |
|---|---|
| Bowel wall thickening (>4 mm) | 100 |
| Peri-colic stranding | 51 |
| Ascites | 43 |
| Bowel dilatation | 38 |
| Mucosal enhancement | 28 |
| Pneumatosis intestinalis | 21 |
| Wall nodularity | 2 |
Table 8.
Summary of CT findings of colitides and enteritides in the immune compromised host.
| Bowel wall thickening (%) | Wall nodularity (%) | Contrast enhancement (%) | Bowel dilatation (%) | Mesenteric stranding (%) | Ascites (%) | Distribution | Normal findings (%) | Discriminating features | |
|---|---|---|---|---|---|---|---|---|---|
| PMC | 86 (11–15 mm) | 38% | 18 (target enhancement) | 14 | 45 (mild) | 38 | Pancolonic (in 46%) | 14 | Accordion sign |
| Typhlitis | 100 (7 mm) | 2% | 28 | 38 | 51 (pronounced) | 43 | Right colon ± small bowel | Pneumatosis intestinalis (20%) | |
| CMV | 92 (15 mm) | 29 (target enhancement) | 92 | 42 | <5% pancolonic, 40% small bowel | 8 | Deep ulceration, adenopathy (16%) | ||
| GVHD | 100 (small bowel), 59 (large bowel) | 54 | 23–86 | 73 | 28–45 | Splenomegaly (36%), peri-portal oedema (36%), GB wall enhancement (23%) |
Cytomegalovirus colitis
Cytomegalovirus (CMV) colitis has been reported most commonly in AIDS patients and in renal transplant patients on immunosuppressive therapy. The infection may involve any part of the gastrointestinal (GI) tract although the colon is most commonly affected. CMV causes a small vessel vasculitis, which may progress to focal bowel wall ischaemia and necrosis.
Plain radiographic appearances are non-specific. CT can help to distinguish CMV colitis from other colitides and is useful in detecting complications such as perforation or abscess formation.
CT appearances of CMV colitis[16] (see Table 6)
Table 6.
CT findings in CMV colitis[16].
| CT findings | Frequency (%) |
|---|---|
| Peri-colic stranding | 92 |
| Bowel wall thickening (>4 mm) | 92 |
| Deep mural ulceration | 63 |
| Mural oedema | 63 |
| Ascites | 42 |
| Target sign | 29 |
| Lymphadenopathy | 16 |
| Normal findings | 8 |
Bowel wall thickening with mesenteric stranding is the most common CT finding. Mean wall thicknesses of 15 mm have been reported, similar to that seen in PMC. Mural thickening is circumferential in the majority of cases and may affect any segment of the large bowel. In contrast to PMC, it rarely results in a pancolitis (<5%). Small bowel involvement is seen in 40% of cases. This is a useful feature in differentiating CMV colitis from PMC, where changes are confined to the colon.
The most prominent and characteristic feature of colonic wall thickening in CMV colitis is mural oedema with deep ulceration (Fig. 6). These changes are thought to be due to occlusive vasculitis causing tissue necrosis progressing to ulceration, haemorrhage and perforation.
Figure 6.
Caecal ulceration in a patient with AIDS. CMV colitis was confirmed by colonoscopic biopsy. Axial MDCT shows marked asymmetric thickening of the caecum with deep mural ulceration (arrows).
Enteritis
Mycobacterium avium intracellulare (MAI)
Disseminated MAI is one of the most commonly encountered opportunistic infections in the AIDS population. Presenting symptoms include severe abdominal pain and systemic symptoms including fever, night sweats and weight loss. The jejunum is the most common site of involvement, but the entire small bowel may be involved. MAI infects Peyer's patches, lymphoid tissue and mesenteric lymph nodes.
CT may reveal bowel wall thickening with prominent nodular mucosal folds, small bowel dilatation and bulky mesenteric and retroperitoneal lymphadenopathy (Fig. 7). In patients with disseminated infection, there may be hepatosplenomegaly with multifocal microabscesses involving the liver, spleen and kidney. The main differential diagnosis for these appearances is lymphoma.
Figure 7.
MAI enteritis. Axial MDCT image of a patient with AIDS presenting with severe abdominal pain and diarrhoea. Note the irregular thickening of the small bowel folds (arrows) and multiple low density mesenteric and retroperitoneal nodes (arrowheads).
Mycobacterium tuberculosis
Tuberculosis of the GI tract affects the ileocaecal area in 90% of cases. Presenting features typically include abdominal pain, chronic diarrhoea, fever and a right iliac fossa mass. Advanced cases may present with bowel obstruction, haemorrhage, and rarely, bowel perforation.
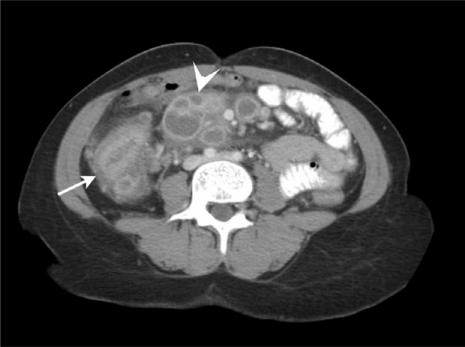
CT characteristically shows prominent asymmetric thickening of the caecum in conjunction with multiple low density enlarged lymph nodes (Fig. 8). The adenopathy predominantly involves mesenteric regions near the diseased intestinal segments and shows both peripheral contrast enhancement and hypodense centres due to caseous necrosis.
Figure 8.
Ileocaecal tuberculosis in a patient with AIDS. Note the thickened caecum (arrow) in conjunction with multiple mesenteric lymph nodes showing peripheral contrast enhancement and hypodense centres (arrowhead).
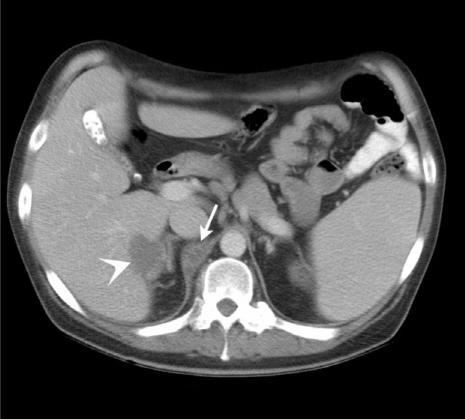
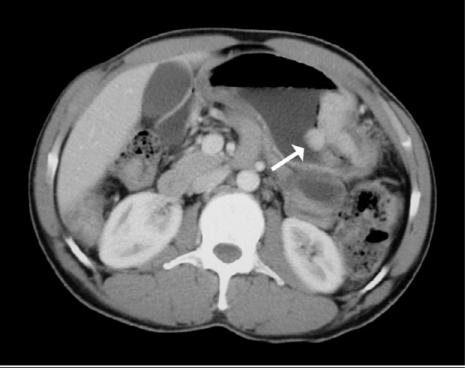
CT may also show additional abdominal findings to support a diagnosis of tuberculosis. Visceral lesions may be noted in the spleen or liver (Fig. 9). Peritoneal involvement can manifest as loculated hyperdense ascitic fluid and peritoneal thickening.
Figure 9.
Tuberculosis. Axial MDCT of a patient with AIDS presenting with abdominal pain and fever. Note the low density retrocrural adenopathy (arrow) and focal hepatic lesion (arrowhead) due to Mycobacterium tuberculosis.
Acute graft-versus-host disease
Graft-versus-host disease (GVHD) occurs when functionally competent T-lymphocytes are introduced into an immunocompromised recipient. Acute GVHD presents within the first 100 days of allogenic bone marrow transplantation. Clinically significant GVHD occurs in 15–50% of adults who receive an allogenic bone marrow transplant[17]. The skin, GI tract and liver are the principally targeted organs. The presenting abdominal symptoms are non-specific and thus CT of the abdomen is frequently performed.
Bowel wall thickening is the most common abnormality. This may be discontinuous and affect both the small and large bowel. Bowel loop dilatation is found proximal to the thickened bowel wall segments. Small bowel is always involved. The CT findings are summarised in Table 7.
Table 7.
CT findings in acute GVHD[18].
| CT findings | Frequency (%) |
|---|---|
| Small bowel wall thickening | 100 |
| Large bowel wall thickening | 59 |
| Mesenteric stranding | 73 |
| Mucosal contrast enhancement | 54 |
| Ascites | 45 |
| Splenomegaly | 36 |
| Peri-portal oedema | 36 |
| Gallbladder wall enhancement | 23 |
| Bowel loop dilatation | 23 |
Mucosal contrast enhancement is seen in just over 50% of cases and mesenteric stranding adjacent to the thickened segments in 75%. Engorgement of the vasa recta is typical. Lymphadenopathy is unusual in GVHD[18]. Extra-intestinal findings include gallbladder wall thickening and enhancement, peri-portal and peri-cholecystic fluid and hepatosplenomegaly.
Other intra-abdominal pathology
Small and large bowel
Bowel perforation
Perforation of small bowel lymphomas is typical of advanced disease or may occur with induction chemotherapy, particularly cytosine arabinoside. Activation of tuberculosis during post-transplant immunosuppressive therapy has been reported to result in small bowel perforation. The deep ulceration caused by CMV infection may also lead to perforation. Colonic perforation in the immunocompromised host has been described following typhlitis, CMV colitis, candidiasis and ischaemic colitis. The CT findings in bowel perforation are:
pneumoperitoneum
abscess or focal collection of extramural fluid and/or air next to perforation site
phlegmon or inflammatory soft tissue mass adjacent to perforation site
mesenteric stranding adjacent to site of perforation
abnormal segment of bowel with wall thickening or pneumatosis
leakage of luminal contrast material at perforation site.
Bowel obstruction
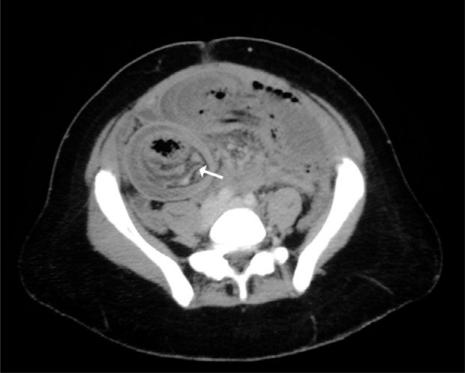
CT is useful for indicating the level of bowel obstruction and demonstrating the cause. The principal cause in these patients, as in the general population, is post-surgical adhesions. Obstruction can also be due to the primary tumour mass or peritoneal carcinomatosis. In AIDS patients, involvement of the bowel by Kaposi's sarcoma and lymphoma should be considered. Both lymphomatous masses and Kaposi sarcoma can serve as lead points for intussusception (Fig. 10).
Figure 10.
Intussusception in an AIDS patient presenting with intermittent abdominal pain and distension. Axial MDCT shows the intussusception with invaginated mesenteric fat accompanying the intussusceptum (arrow).
Kaposi's sarcoma (KS) is a common primary clinical manifestation of AIDS. In these patients, KS rarely presents in the GI tract without associated cutaneous manifestations. Mural lesions are seen in around 40% of AIDS patients with KS (Fig. 11). Whilst usually asymptomatic, they may rarely cause GI haemorrhage, obstruction or act as a lead point of an intussusception.
Figure 11.
Axial MDCT image of a patient with AIDS shows a large enhancing polypoid lesion arising from the wall of the stomach (arrow). Following endoscopic biopsy, this was found to be Kaposi's sarcoma.
On CT, mural lesions may present as focal target lesions or with more diffuse mural thickening. Although KS affects the liver and spleen in a third of patients with cutaneous disease, involvement is rarely demonstrated on CT. Lymph nodes involved with KS have a characteristic appearance on CT. They show bright enhancement after intravenous contrast. This hyperattenuating adenopathy has been shown to have a positive predictive value for KS of 79%[19].
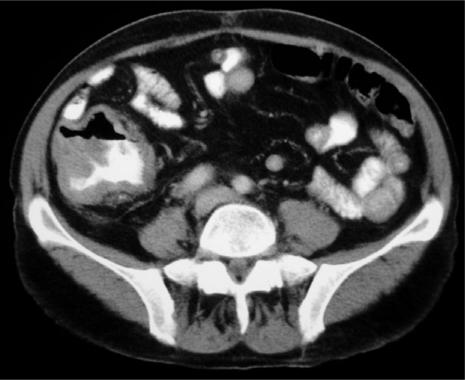
The GI tract is the commonest site of disease with AIDS-related lymphoma (ARL). ARLs are aggressive and have a generally poor prognosis. They commonly affect multiple sites within the abdomen including bowel, lymph nodes and solid organs. This multifocal pattern of involvement makes CT an ideal imaging modality.
In contrast to non-AIDS GI lymphoma, the stomach is less frequently affected in ARL. More distal involvement is common. The affected bowel segment typically has a thickened wall and lesions are often large by the time of presentation. Ulceration may be present (Fig. 12).
Figure 12.
Axial MDCT of an HIV-positive patient presenting with right lower quadrant pain. A discrete caecal mass with ulceration is seen. This proved to be a solitary lymphoma and became the AIDS-defining illness.
Focal splenic and hepatic involvement is more common in ARL patients than in the non-AIDS lymphoma population. Hepatic lesions are typically 1–3 cm in size, rounded and have an attenuation value slightly less than that of contrast-enhanced hepatic parenchyma.
Stomach
Gastric pathology may present with pain, bleeding, perforation or obstruction. Causes of upper GI haemorrhage in the immunocompromised population include CMV, lymphoma, Kaposi's sarcoma (Fig. 12) and Candida.
Corticosteroid and anti-metabolite drugs may contribute to the ulcer formation. Coagulation abnormalities are common in this patient population and further contribute to the bleeding diathesis.
Hepaticobiliary and pancreatic sites
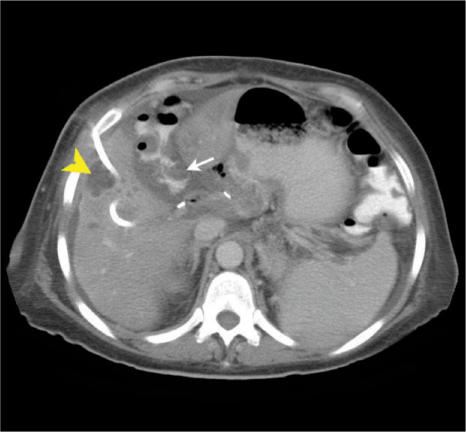
The incidence of acalculous cholecystitis is increased in the immunocompromised population. Atypical organisms such as CMV, Cryptosporidium and Listeria are causative. Imaging reveals a thickened gallbladder wall, oedema and peri-cholecystic fluid. Pancreatitis is relatively rare in this patient population but carries a high mortality rate. Hypercalcaemia, CMV infection and medications such as corticosteroids and azathioprine are possible aetiologies. Typical CT findings include focal or diffuse enlargement of the pancreas, decrease in the density of the parenchyma due to oedema and blurring of the margins due to inflammation. Peri-pancreatic fat stranding and thickening of retroperitoneal fascia involved may also be observed. Pyogenic liver abscesses may present with right upper quadrant pain. Often they are clinically silent. The differential diagnosis includes lymphoma, Kaposi's sarcoma and peliosis hepatitis. Percutaneous aspiration and tube drainage is the procedure of choice (Fig. 13). Fungal micro-abscesses may be seen in patients on chemotherapy, steroids or those with neutropenia. Similar pyogenic and fungal abscesses may occur in the spleen. Pathogens include atypical mycobacteria, tuberculosis, Candida and Pneumocystis carinii. Right upper quadrant pain, fever and jaundice may be an early manifestation of GVHD in the bone marrow transplant population. Hepatic GVHD and the use of parenteral nutrition in this group leads to cholestasis, contributing to the development of gallbladder sludge and calculi.
Figure 13.
Patient with PMC (as seen in Fig. 4) and a pyogenic liver abscess (arrowhead). Note the presence of the accordion sign due to PMC (arrow). The liver abscess has a percutaneous drain in situ.
References
- [1].Chatila W, Manthous CA. Clostridium difficile causing sepsis and an acute abdomen in critically ill patients. Crit Care Med. 1995;23:1146–50. doi: 10.1097/00003246-199506000-00024. [DOI] [PubMed] [Google Scholar]
- [2].Jobe BA, Grasley A, Deveney KE, Deveney CW, Sheppard BC. Clostridium difficile colitis: an increasing hospital-acquired illness. Am J Surg. 1995;169:480–3. doi: 10.1016/S0002-9610(99)80199-8. [DOI] [PubMed] [Google Scholar]
- [3].Bowland GW, Lee NG, Cats A, Mueller PR. Pseudomembranous colitis: diagnostic sensitivity of the abdominal plain radiograph. Clin Radiol. 1994;49:473–5. doi: 10.1016/s0009-9260(05)81744-1. [DOI] [PubMed] [Google Scholar]
- [4].Downey DB, Wilson SR. Pseudomembranous colitis: sonographic features. Radiology. 1991;180:61–4. doi: 10.1148/radiology.180.1.2052724. [DOI] [PubMed] [Google Scholar]
- [5].Ramachandran I, Sinha R, Rodgers P. Pseudomembranous colitis revisited: spectrum of imaging findings. Clin Radiol. 2006;61:535–44. doi: 10.1016/j.crad.2006.03.009. [DOI] [PubMed] [Google Scholar]
- [6].Fishman EK, Kavuru M, Jones B. Pseudomembranous colitis: CT evaluation of 26 cases. Radiology. 1996;180:57–60. doi: 10.1148/radiology.180.1.2052723. [DOI] [PubMed] [Google Scholar]
- [7].Kawamoto S, Horton KM, Fishman EK. Pseudomembranous colitis: spectrum of imaging findings with clinical and pathologic correlation. Radiographics. 1999;19:887–97. doi: 10.1148/radiographics.19.4.g99jl07887. [DOI] [PubMed] [Google Scholar]
- [8].Horton KM, Corl FM, Fishman EK. CT evaluation of the colon: inflammatory disease. RadioGraphics. 2000;20:399–418. doi: 10.1148/radiographics.20.2.g00mc15399. [DOI] [PubMed] [Google Scholar]
- [9].Macari M, Balthazar EJ. CT of bowel wall thickening: significance and pitfalls of interpretation. AJR. 2001;176:1105–16. doi: 10.2214/ajr.176.5.1761105. [DOI] [PubMed] [Google Scholar]
- [10].Ahualli J. The target sign: bowel wall. Radiology. 2005;234:549–50. doi: 10.1148/radiol.2342031015. [DOI] [PubMed] [Google Scholar]
- [11].Kirkpatrick I, Greenberg HM. Evaluating the CT diagnosis of Clostridium difficile colitis: should CT guide therapy? AJR. 2000;176:635–9. doi: 10.2214/ajr.176.3.1760635. [DOI] [PubMed] [Google Scholar]
- [12].Macari M, Balthazar EJ, Megibow AJ. The accordion sign at CT: a non-specific finding in patients with colonic edema. Radiology. 1999;211:743–6. doi: 10.1148/radiology.211.3.r99jn32743. [DOI] [PubMed] [Google Scholar]
- [13].Urbach DR, Rotstein OD. Typhlitis. Can J Surg. 1999;42:415–19. [PMC free article] [PubMed] [Google Scholar]
- [14].Kirkpatrick I, Greenberg HM. Gastrointestinal complications in the neutropenic patient: characterization and differentiation with abdominal CT. Radiology. 2003;226:668–74. doi: 10.1148/radiol.2263011932. [DOI] [PubMed] [Google Scholar]
- [15].Davila ML. Neutropenic enterocolitis. Curr Opin Gastroenterol. 2006;22:44–7. doi: 10.1097/01.mog.0000198073.14169.3b. [DOI] [PubMed] [Google Scholar]
- [16].Murray JG, Evans SJ, Jeffrey PB, Halvorsen RA. Cytomegalovirus colitis in AIDS: CT features. AJR. 1995;165:67–71. doi: 10.2214/ajr.165.1.7785636. [DOI] [PubMed] [Google Scholar]
- [17].Thomas ED. Bone marrow transplantation: a review. Semin Haematol. 1999;36:95–103. [PubMed] [Google Scholar]
- [18].Kalantari BN, Mortele KJ, Cantisani V, Ondategui S. CT features with pathologic correlation of acute graft-versus-host disease after bone marrow transplantation in adults. AJR. 2003;181:1621–6. doi: 10.2214/ajr.181.6.1811621. [DOI] [PubMed] [Google Scholar]
- [19].Herts BR, Megibow AJ, Birnbaum BA, et al. High attenuation lymphadenopathy in AIDS patients: significance of findings at CT. Radiology. 1992;185:777–81. doi: 10.1148/radiology.185.3.1438762. [DOI] [PubMed] [Google Scholar]