Figure 1. G- but not F-actin is a substrate for the ACD-induced cross-linking.
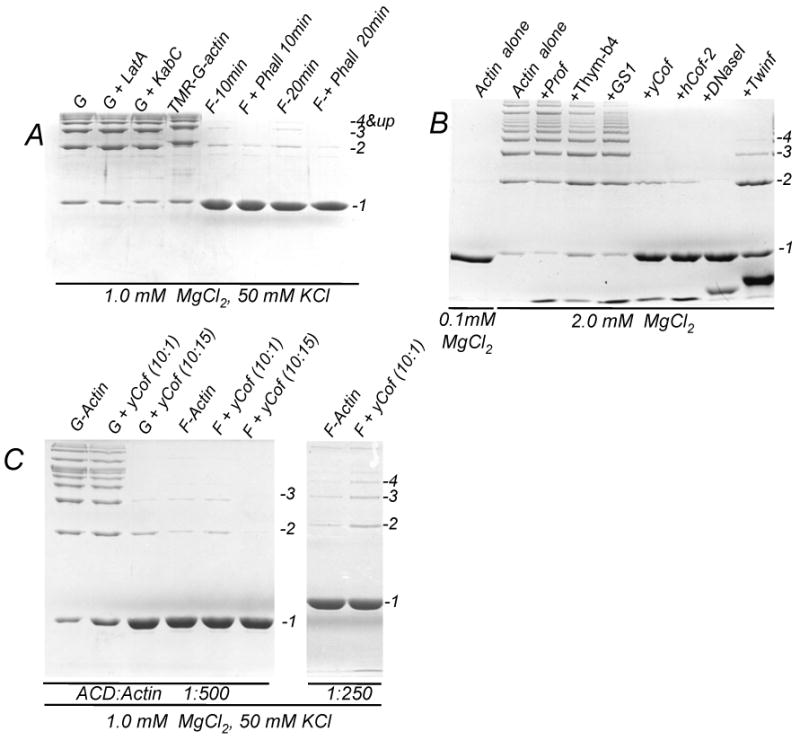
(A) Cross-linking of 10 μM actin in G- (G) and F-(F) states was initiated by adding LFNACD (1 to 500 mole ratio of ACD to actin) in the presence of 1.0 mM MgCl2 and 50 mM KCl. TMR-labeled actin (TMR-G-actin), as well as actin in the presence of Lat A (G+LatA) and KabC (G+KabC) were used to block the polymerization of Mg-G-actin into filaments. Phalloidin (15 μM) was added to F-actin (F+Phall) to inhibit filaments treadmilling. The extent of the cross-linking was assessed after 10 min incubation with LFNACD. For F-actin, even after 20 min incubation only a small amount of actin was cross-linked (F-20min and F+Phall 20 min), likely as a result of treadmilling. The cross-linking reaction was stopped with a sample buffer and analyzed by 10% SDS-PAGE.
(B) Actin alone and in the presence of ABPs was cross-linked for 15 minutes in the presence 2.0 mM MgCl2 at 1 to 500 mole ratio of LFNACD to actin. No cross-linking was observed in the presence of 0.1 mM MgCl2 (left lane). The ABPs are annotated as follows: profilin (+Prof), thymosin-β4 (+Thym-β4), gelsolin segment 1 (+GS1), yeast cofilin (+YCof), human cofilin-2 (HCof-2), DNaseI (+DNaseI), twinfilin with truncated C-terminus (+Twinf). Full length twinfilin showed the same inhibitory effect as its shortened counterpart (data not shown).
(C) 10 μM G-actin and 10 μM F-actin, polymerized for 2 hours before the cross-linking, was pre-incubated for 20 minutes with 1.0 μM or 15 μM yeast cofilin. The cross-linking reaction was initiated by adding LFNACD at 1 to 500 (left panel) or 1 to 250 (right panel) mole ratio to actin and stopped after 30 minutes (left panel) or 60 minutes (right panel) after the initiation.
