Figure 4. Solution ATP, and not ATP in the nucleotide cleft of actin, is required for actin cross-linking with LFNACD.
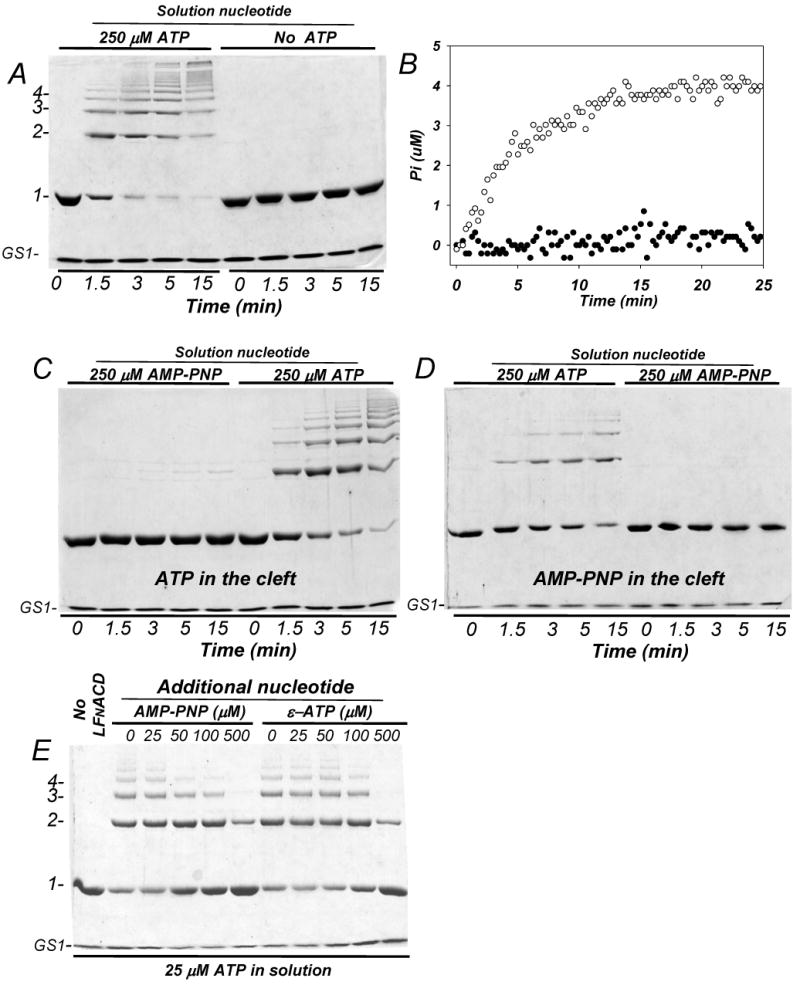
(A) The cross-linking of 9 μM Mg-G-actin-GS1 complex with LFNACD (1 to 250 mole ratio) was conducted in the absence and the presence of 250 μM ATP in the reaction solution. The cross-linking reaction was initiated by adding 1.0 mM MgCl2. (B) The cross-linking of 5 μM ATP-G-actin-GS1 complex with 0.02 μM LFNACD (1 to 250 mole ratio to actin) in the presence (open circles) and absence (closed circles) of 100 μM free ATP in solution was measured with the EnzChek Phosphate kit. Neither cross-linking (A) nor Pi release (B) occurred in the absence of free ATP in the solution. (C) A complex of ATP-actin and GS1 (10 and 12.5 μM final concentrations, respectively) was transferred into the buffer free of ATP via a PD10 size exclusion column and then supplemented with 250 μM of either AMP-PNP or ATP (as indicated). The cross-linking was initiated by adding 0.04 μM of LFNACD and 1.0 mM MgCl2. (D) A complex of GS1 (7.5 μM) with G-actin (6.0 μM) with AMP-PNP bound at the nucleotide binding cleft was supplemented with 250 μM of either ATP or AMP-PNP and 0.024 μM LFNACD. (C) and (D) show that free ATP in solution is required to support the cross-linking, while either ATP or non-hydrolysable AMP-PNP bound to the actin nucleotide-binding cleft allow the reaction. (E) ATP-Actin (10 μM) in complex with GS1 (15 μM) was cross-linked for 10 minutes with 0.04 μM of LFNACD (1 to 250 mole ratio to actin) in the presence of 25 μM free ATP and increasing concentrations of either AMP-PNP or εATP. Although εATP can be hydrolyzed to Pi and εADP by some enzymes, ACD dose not appear to use it as an energy source.
