Abstract
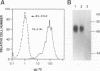
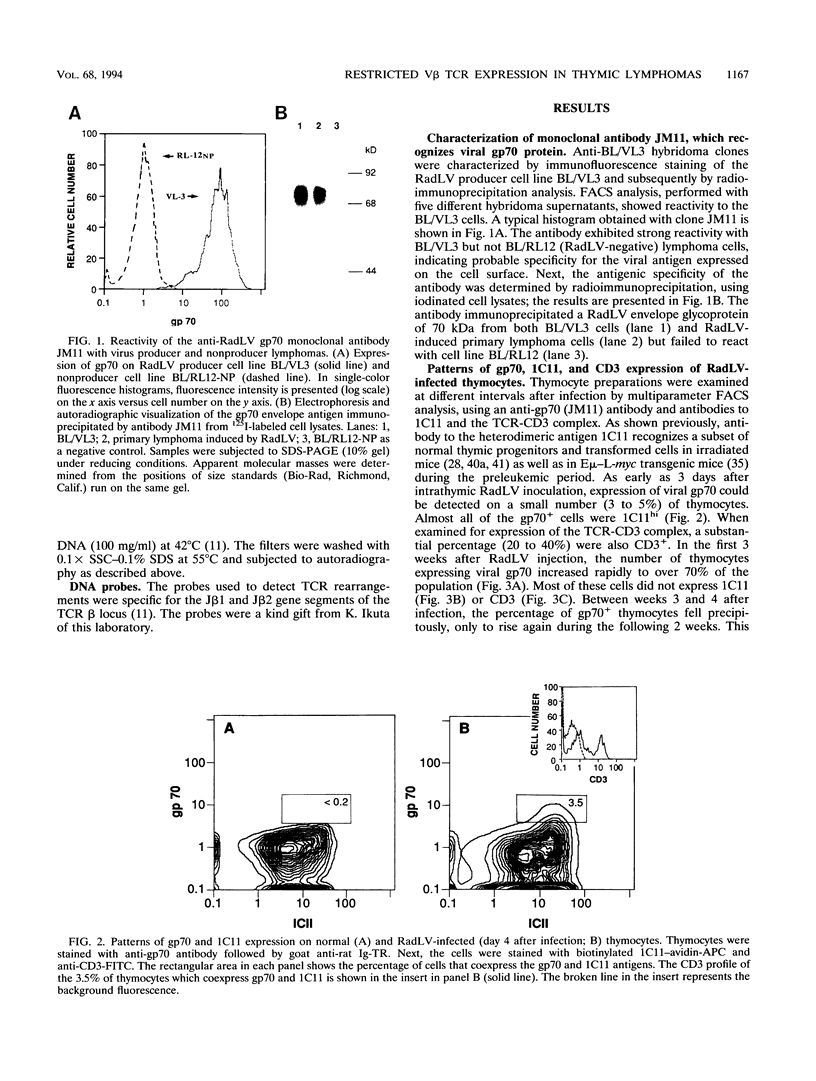
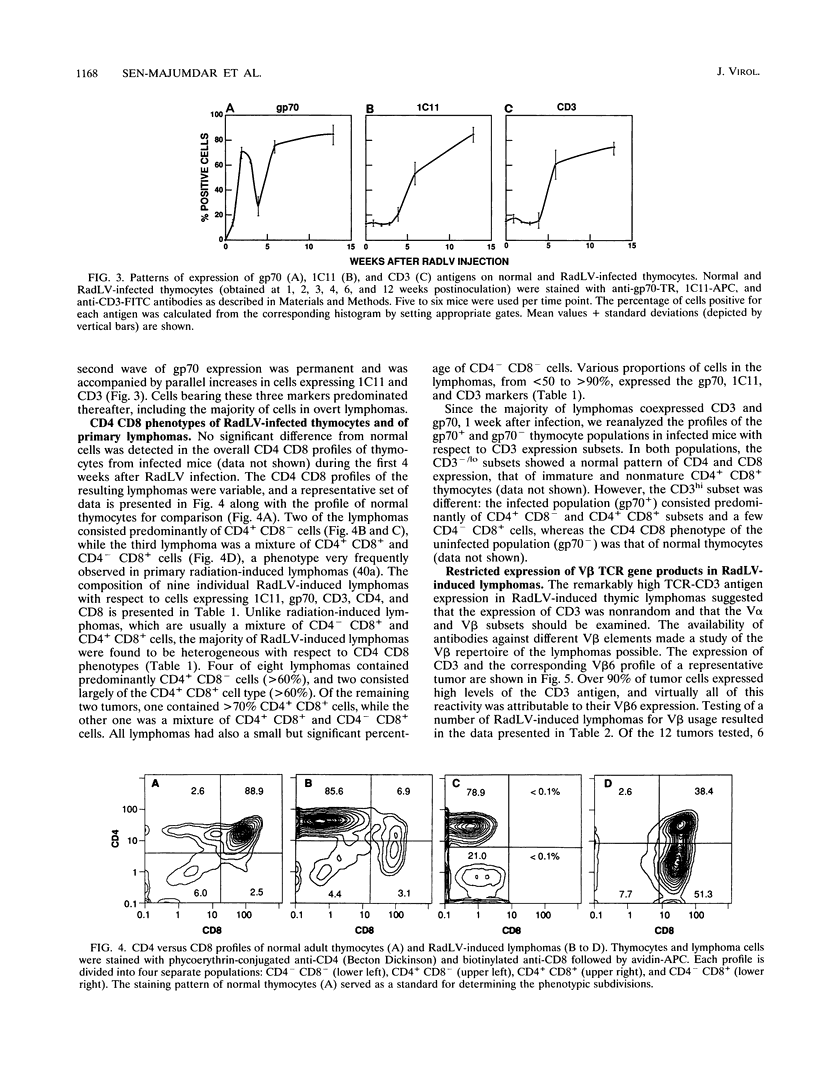
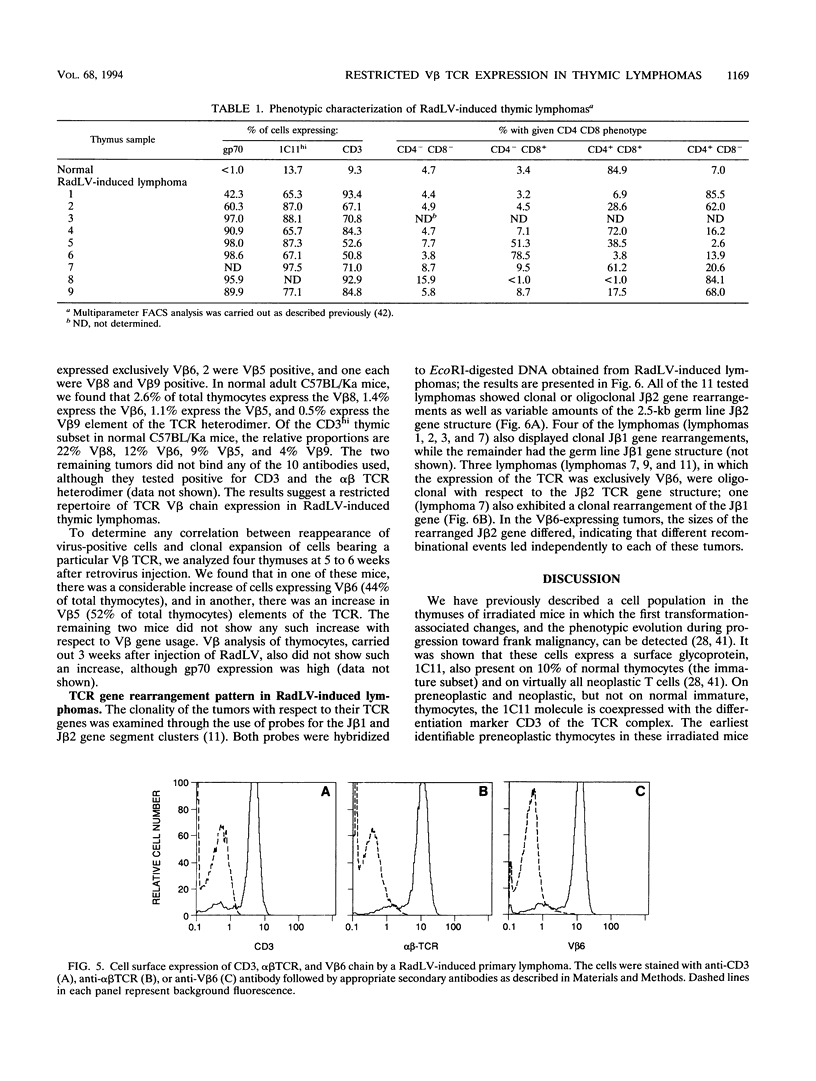
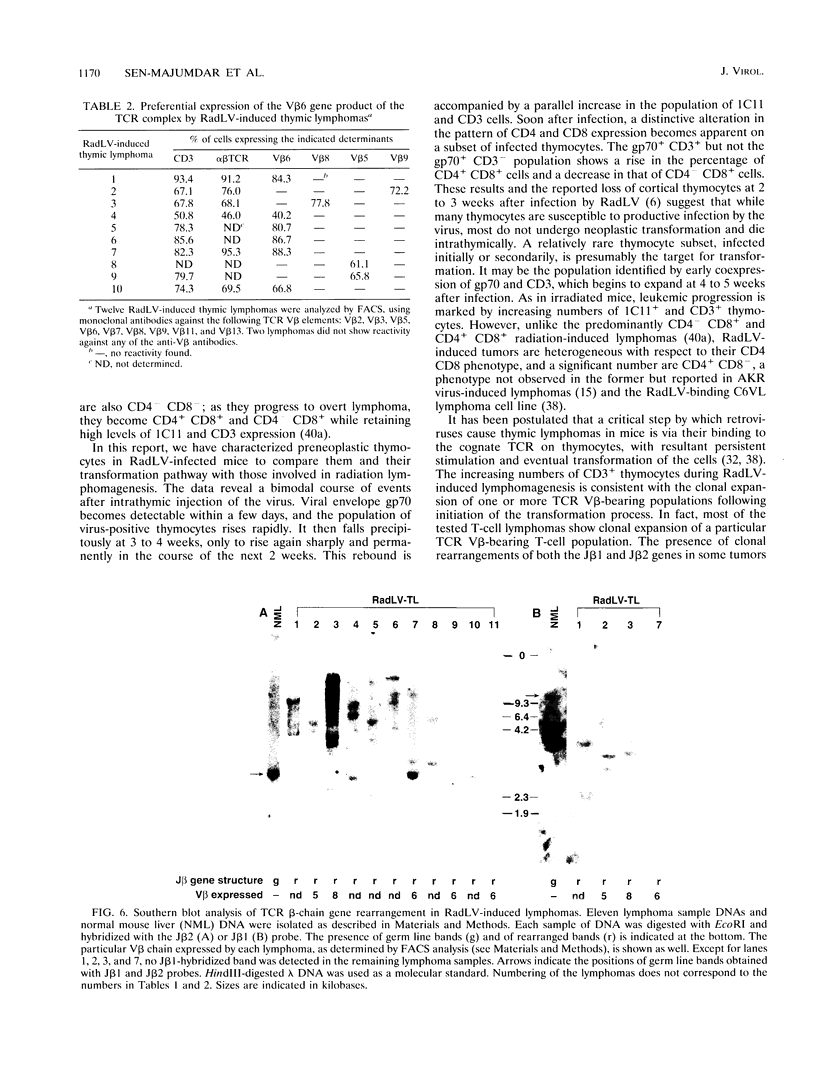
We have investigated the phenotypic changes that take place during the process of neoplastic transformation in the thymocytes of C57BL/Ka mice infected by the radiation leukemia virus (RadLV). By the combined use of antibodies against the envelope glycoprotein gp70 of RadLV, the transformation-associated cell surface marker 1C11, and the CD3-T-cell receptor (TCR) complex, we found that in the RadLV-infected thymus, the earliest expression of viral gp70 is in 1C11hi cells; a small but significant percentage of these cells also express CD3. A first wave of viral replication, manifested by the expression of high levels of gp70 in thymocytes (over 70% positive), reaches a peak at 2 weeks; during this period, no significant changes are observed in the expression of 1C11 or CD3. The population of gp70+ cells is drastically reduced at 3 to 4 weeks after infection. However, a second cohort of gp70+ cells appears after 4 weeks, and these cells express high levels of 1C11 and TCR determinants as well. RadLV-induced lymphomas differ from normal thymocytes in their CD4 CD8 phenotype, with domination by one or more subsets. Characterization of TCR gene rearrangements in RadLV-induced lymphomas shows that most of these tumors are clonal or oligoclonal with respect to the J beta 2 TCR gene, while the J beta 1 TCR gene is rearranged in a minority (4 of 11) of lymphomas. TCR V beta repertoire analysis of 12 tumors reveals that 6 (50%) express exclusively the V beta 6 gene product, 2 (17%) are V beta 5+, and 1 (8%) each are V beta 8+ and V beta 9+. In normal C57BL/Ka mice, V beta 6 is expressed on 12%, V beta 5 is expressed on 9%, V beta 8 is expressed on 22%, and V beta 9 is expressed on 4% of TCRhi thymocytes. Thus, it appears that RadLV-induced thymic lymphomas are not randomly selected with respect to expressed TCR V beta type.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acha-Orbea H., Mitchell D. J., Timmermann L., Wraith D. C., Tausch G. S., Waldor M. K., Zamvil S. S., McDevitt H. O., Steinman L. Limited heterogeneity of T cell receptors from lymphocytes mediating autoimmune encephalomyelitis allows specific immune intervention. Cell. 1988 Jul 15;54(2):263–273. doi: 10.1016/0092-8674(88)90558-2. [DOI] [PubMed] [Google Scholar]
- Acha-Orbea H., Shakhov A. N., Scarpellino L., Kolb E., Müller V., Vessaz-Shaw A., Fuchs R., Blöchlinger K., Rollini P., Billotte J. Clonal deletion of V beta 14-bearing T cells in mice transgenic for mammary tumour virus. Nature. 1991 Mar 21;350(6315):207–211. doi: 10.1038/350207a0. [DOI] [PubMed] [Google Scholar]
- Adkins B., Mueller C., Okada C. Y., Reichert R. A., Weissman I. L., Spangrude G. J. Early events in T-cell maturation. Annu Rev Immunol. 1987;5:325–365. doi: 10.1146/annurev.iy.05.040187.001545. [DOI] [PubMed] [Google Scholar]
- Benade L. E., Ihle J. N., Declève A. Serological characterization of B-tropic viruses of C57BL mice: possible origin by recombination of endogenous N-tropic and xenotropic viruses. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4553–4557. doi: 10.1073/pnas.75.9.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bill J., Kanagawa O., Woodland D. L., Palmer E. The MHC molecule I-E is necessary but not sufficient for the clonal deletion of V beta 11-bearing T cells. J Exp Med. 1989 Apr 1;169(4):1405–1419. doi: 10.1084/jem.169.4.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boniver J., Declève A., Honsik C., Lieberman M., Kaplan H. S. Phenotypic characterization of mice of thymus target cells susceptible to productive infection by the radiation leukemia virus. J Natl Cancer Inst. 1981 Nov;67(5):1139–1151. [PubMed] [Google Scholar]
- Davis M. M., Bjorkman P. J. T-cell antigen receptor genes and T-cell recognition. Nature. 1988 Aug 4;334(6181):395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- DesGroseillers L., Jolicoeur P. The tandem direct repeats within the long terminal repeat of murine leukemia viruses are the primary determinant of their leukemogenic potential. J Virol. 1984 Dec;52(3):945–952. doi: 10.1128/jvi.52.3.945-952.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famulari N. G. Murine leukemia viruses with recombinant env genes: a discussion of their role in leukemogenesis. Curr Top Microbiol Immunol. 1983;103:75–108. doi: 10.1007/978-3-642-68943-7_4. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gascoigne N. R., Chien Y., Becker D. M., Kavaler J., Davis M. M. Genomic organization and sequence of T-cell receptor beta-chain constant- and joining-region genes. Nature. 1984 Aug 2;310(5976):387–391. doi: 10.1038/310387a0. [DOI] [PubMed] [Google Scholar]
- Grégoire C., Rebaï N., Schweisguth F., Necker A., Mazza G., Auphan N., Millward A., Schmitt-Verhulst A. M., Malissen B. Engineered secreted T-cell receptor alpha beta heterodimers. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8077–8081. doi: 10.1073/pnas.88.18.8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidos C. J., Danska J. S., Fathman C. G., Weissman I. L. T cell receptor-mediated negative selection of autoreactive T lymphocyte precursors occurs after commitment to the CD4 or CD8 lineages. J Exp Med. 1990 Sep 1;172(3):835–845. doi: 10.1084/jem.172.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidos C. J., Weissman I. L., Adkins B. Intrathymic maturation of murine T lymphocytes from CD8+ precursors. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7542–7546. doi: 10.1073/pnas.86.19.7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward W. S., Neel B. G., Astrin S. M. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosis. Nature. 1981 Apr 9;290(5806):475–480. doi: 10.1038/290475a0. [DOI] [PubMed] [Google Scholar]
- Holland C. A., Hartley J. W., Rowe W. P., Hopkins N. At least four viral genes contribute to the leukemogenicity of murine retrovirus MCF 247 in AKR mice. J Virol. 1985 Jan;53(1):158–165. doi: 10.1128/jvi.53.1.158-165.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hügin A. W., Vacchio M. S., Morse H. C., 3rd A virus-encoded "superantigen" in a retrovirus-induced immunodeficiency syndrome of mice. Science. 1991 Apr 19;252(5004):424–427. doi: 10.1126/science.1850169. [DOI] [PubMed] [Google Scholar]
- Infante A. J., Levcovitz H., Gordon V., Wall K. A., Thompson P. A., Krolick K. A. Preferential use of a T cell receptor V beta gene by acetylcholine receptor reactive T cells from myasthenia gravis-susceptible mice. J Immunol. 1992 Jun 1;148(11):3385–3390. [PubMed] [Google Scholar]
- KAPLAN H. S., BROWN M. B., PAULL J. Influence of bone-marrow injections on involution and neoplasia of mouse thymus after systemic irradiation. J Natl Cancer Inst. 1953 Oct;14(2):303–316. doi: 10.1093/jnci/14.2.303. [DOI] [PubMed] [Google Scholar]
- Kanagawa O., Palmer E., Bill J. The T cell receptor V beta 6 domain imparts reactivity to the Mls-1a antigen. Cell Immunol. 1989 Apr 1;119(2):412–426. doi: 10.1016/0008-8749(89)90255-4. [DOI] [PubMed] [Google Scholar]
- Kaplan H. S. On the natural history of the murine leukemias: presidential address. Cancer Res. 1967 Aug;27(8):1325–1340. [PubMed] [Google Scholar]
- Kappler J. W., Wade T., White J., Kushnir E., Blackman M., Bill J., Roehm N., Marrack P. A T cell receptor V beta segment that imparts reactivity to a class II major histocompatibility complex product. Cell. 1987 Apr 24;49(2):263–271. doi: 10.1016/0092-8674(87)90567-8. [DOI] [PubMed] [Google Scholar]
- Khan A. S., Martin M. A. Endogenous murine leukemia proviral long terminal repeats contain a unique 190-base-pair insert. Proc Natl Acad Sci U S A. 1983 May;80(9):2699–2703. doi: 10.1073/pnas.80.9.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIEBERMAN M., KAPLAN H. S. Leukemogenic activity of filtrates from radiation-induced lymphoid tumors of mice. Science. 1959 Aug 14;130(3372):387–388. doi: 10.1126/science.130.3372.387. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Lenz J., Celander D., Crowther R. L., Patarca R., Perkins D. W., Haseltine W. A. Determination of the leukaemogenicity of a murine retrovirus by sequences within the long terminal repeat. 1984 Mar 29-Apr 4Nature. 308(5958):467–470. doi: 10.1038/308467a0. [DOI] [PubMed] [Google Scholar]
- Lieberman M., Declève A., Ricciardi-Castagnoli P., Boniver J., Finn O. J., Kaplan H. S. Establishment, characterization and virus expression of cell lines derived from radiation- and virus-induced lymphomas of C57BL/Ka mice. Int J Cancer. 1979 Aug;24(2):168–177. doi: 10.1002/ijc.2910240208. [DOI] [PubMed] [Google Scholar]
- Lieberman M., Hansteen G. A., Waller E. K., Weissman I. L., Sen-Majumdar A. Unexpected effects of the severe combined immunodeficiency mutation on murine lymphomagenesis. J Exp Med. 1992 Aug 1;176(2):399–405. doi: 10.1084/jem.176.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar A. S., Guidos C., Kaneshima H., White J. H., Marian J., Lieberman M., Weissman I. L. An immunodominant murine lymphoma cell surface heterodimer marks thymic progenitor subsets. J Immunol. 1990 Jan 1;144(1):111–121. [PubMed] [Google Scholar]
- Manteuil-Brutlag S., Liu S. L., Kaplan H. S. Radiation leukemia virus contains two distinct viral RNAs. Cell. 1980 Mar;19(3):643–652. doi: 10.1016/s0092-8674(80)80041-9. [DOI] [PubMed] [Google Scholar]
- Marrack P., Kushnir E., Kappler J. A maternally inherited superantigen encoded by a mammary tumour virus. Nature. 1991 Feb 7;349(6309):524–526. doi: 10.1038/349524a0. [DOI] [PubMed] [Google Scholar]
- McGrath M. S., Weissman I. L. AKR leukemogenesis: identification and biological significance of thymic lymphoma receptors for AKR retroviruses. Cell. 1979 May;17(1):65–75. doi: 10.1016/0092-8674(79)90295-2. [DOI] [PubMed] [Google Scholar]
- Merregaert J., Janowski M., Reddy E. P. Nucleotide sequence of a radiation leukemia virus genome. Virology. 1987 May;158(1):88–102. doi: 10.1016/0042-6822(87)90241-8. [DOI] [PubMed] [Google Scholar]
- Möröy T., Fisher P., Guidos C., Ma A., Zimmerman K., Tesfaye A., DePinho R., Weissman I., Alt F. W. IgH enhancer deregulated expression of L-myc: abnormal T lymphocyte development and T cell lymphomagenesis. EMBO J. 1990 Nov;9(11):3659–3666. doi: 10.1002/j.1460-2075.1990.tb07577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitta T., Oksenberg J. R., Rao N. A., Steinman L. Predominant expression of T cell receptor V alpha 7 in tumor-infiltrating lymphocytes of uveal melanoma. Science. 1990 Aug 10;249(4969):672–674. doi: 10.1126/science.2382141. [DOI] [PubMed] [Google Scholar]
- O'Neill H. C., McGrath M. S., Allison J. P., Weissman I. L. A subset of T cell receptors associated with L3T4 molecules mediates C6VL leukemia cell binding of its cognate retrovirus. Cell. 1987 Apr 10;49(1):143–151. doi: 10.1016/0092-8674(87)90764-1. [DOI] [PubMed] [Google Scholar]
- Okada C. Y., Holzmann B., Guidos C., Palmer E., Weissman I. L. Characterization of a rat monoclonal antibody specific for a determinant encoded by the V beta 7 gene segment. Depletion of V beta 7+ T cells in mice with Mls-1a haplotype. J Immunol. 1990 May 1;144(9):3473–3477. [PubMed] [Google Scholar]
- Pullen A. M., Marrack P., Kappler J. W. The T-cell repertoire is heavily influenced by tolerance to polymorphic self-antigens. Nature. 1988 Oct 27;335(6193):796–801. doi: 10.1038/335796a0. [DOI] [PubMed] [Google Scholar]
- Reich E. P., Sherwin R. S., Kanagawa O., Janeway C. A., Jr An explanation for the protective effect of the MHC class II I-E molecule in murine diabetes. Nature. 1989 Sep 28;341(6240):326–328. doi: 10.1038/341326a0. [DOI] [PubMed] [Google Scholar]
- Sen-Majumdar A., Lieberman M., Alpert S., Wiessman I. L., Small M. Differentiation of CD3-4-8- thymocytes in short-term thymic stromal cell culture. J Exp Med. 1992 Aug 1;176(2):543–551. doi: 10.1084/jem.176.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staerz U. D., Rammensee H. G., Benedetto J. D., Bevan M. J. Characterization of a murine monoclonal antibody specific for an allotypic determinant on T cell antigen receptor. J Immunol. 1985 Jun;134(6):3994–4000. [PubMed] [Google Scholar]
- Suzuki M., Koseki H., Mizutani Y., Kuribayashi K., Kanno M., Taniguchi M. Expansion of murine T cells bearing a unique T cell receptor beta-chain in Friend virus-induced tumor in situ. J Immunol. 1992 May 1;148(9):2968–2973. [PubMed] [Google Scholar]
- Urban J. L., Kumar V., Kono D. H., Gomez C., Horvath S. J., Clayton J., Ando D. G., Sercarz E. E., Hood L. Restricted use of T cell receptor V genes in murine autoimmune encephalomyelitis raises possibilities for antibody therapy. Cell. 1988 Aug 12;54(4):577–592. doi: 10.1016/0092-8674(88)90079-7. [DOI] [PubMed] [Google Scholar]
- Utsunomiya Y., Kosaka H., Kanagawa O. Differential reactivity of V beta 9 T cells to minor lymphocyte stimulating antigen in vitro and in vivo. Eur J Immunol. 1991 Apr;21(4):1007–1011. doi: 10.1002/eji.1830210422. [DOI] [PubMed] [Google Scholar]
- Weissman I. L., McGrath M. S. Retrovirus lymphomagenesis: relationship of normal immune receptors to malignant cell proliferation. Curr Top Microbiol Immunol. 1982;98:103–112. doi: 10.1007/978-3-642-68369-5_8. [DOI] [PubMed] [Google Scholar]
- Zaller D. M., Osman G., Kanagawa O., Hood L. Prevention and treatment of murine experimental allergic encephalomyelitis with T cell receptor V beta-specific antibodies. J Exp Med. 1990 Jun 1;171(6):1943–1955. doi: 10.1084/jem.171.6.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamvil S. S., Mitchell D. J., Lee N. E., Moore A. C., Waldor M. K., Sakai K., Rothbard J. B., McDevitt H. O., Steinman L., Acha-Orbea H. Predominant expression of a T cell receptor V beta gene subfamily in autoimmune encephalomyelitis. J Exp Med. 1988 May 1;167(5):1586–1596. doi: 10.1084/jem.167.5.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]