Fig. 4.
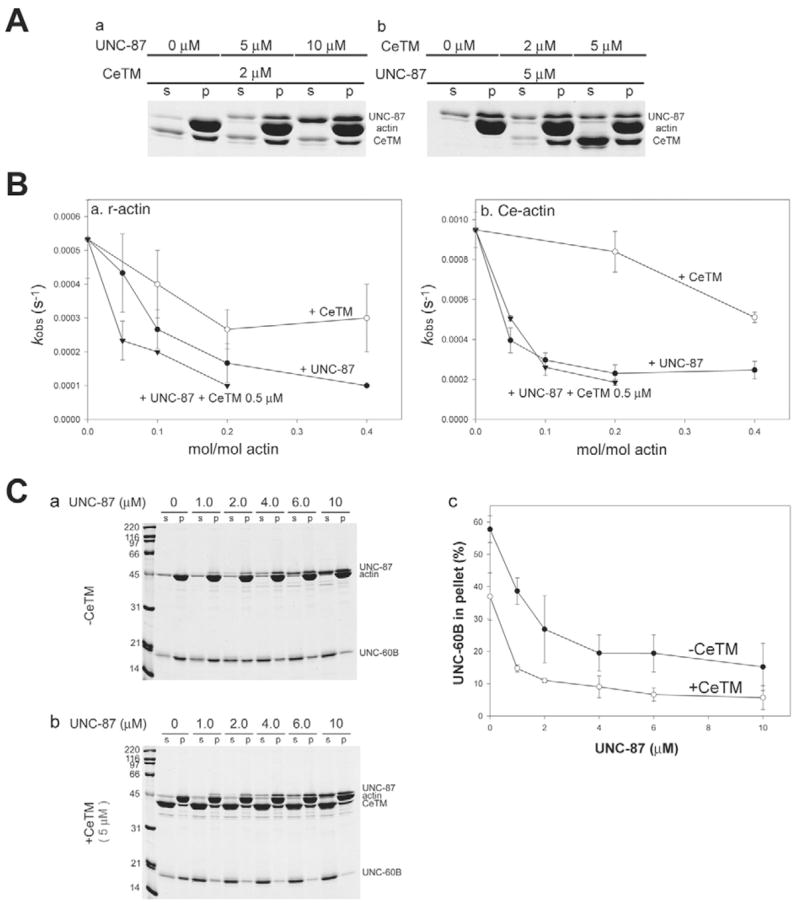
UNC-87 and tropomyosin inhibit UNC-60B-mediated actin turnover. (A) Simultaneous binding of UNC-87 and CeTM to Ce-actin. (a) Ce-actin (10 μM) was pre-incubated with 0–10 μM UNC-87 for 30 minutes and subsequently incubated with 2 μM CeTM for 30 minutes. The reactions were analyzed by pelleting assays (s, supernatants; p, pellets). (b) Ce-actin (10 μM) was pre-incubated with 0–5 μM CeTM for 30 minutes and subsequently incubated with 5 μM UNC-87 for 30 minutes. The reactions were analyzed by pelleting assays as shown in a. (B) Effects of UNC-87 or CeTM on UNC-60B-accelerated actin turnover were measured by nucleotide exchange. F-actin (5 μM) from rabbit muscle (a) or C. elegans (b) was mixed with UNC-60B (2.5 μM) and UNC-87 (0, 0.25, 0.5, 1, or 2 μM; black circles), UNC-60B (2.5 μM) and CeTM (0, 0.5, 1, or 2 μM; white circles), or UNC-60B (2.5 μM), CeTM (0.5 μM) and UNC-87 (0, 0.5, 1, or 2 μM; black triangles) in the presence of 40 μM etheno-ATP, and the fluorescence of etheno-ATP was monitored over time. The data were fitted to exponential curves and the rate of increase in the fluorescence (kobs: 1/second) were calculated and plotted as a function of concentration of UNC-87 or CeTM. Values are mean ± s.d. of three experiments. (C) Co-pelleting assay of 10 μM Ce-actin with 10 μM UNC-60B after pre-incubation with various concentrations of UNC-87 in the absence (a; black circles in c) or presence of 5 μM CeTM (b; white circles in c). Relative amounts (%) of actin-bound UNC-60B in the pellets were plotted as a function of total UNC-87 concentrations (c). Values are means ± s.d. of three experiments.
