Abstract
In September 2006, the US Centers for Disease Control and Prevention (CDC) released new guidelines calling for routine, voluntary human immunodeficiency virus (HIV) testing for all persons aged 13–64 years in health care settings. These guidelines were motivated, in part, by mounting evidence that the traditional approach of using risk factors to identify candidates for HIV testing is inadequate. Of the 1.0–1.2 million people in the United States thought to be infected with HIV, ~25% remain unaware of their infection, and nearly half of all infected patients develop acquired immunodeficiency syndrome ≤1 year after testing positive for HIV. Also contributing to the change in testing guidelines was recent evidence that routine HIV testing is cost-effective. Cost-effectiveness analysis, a method of assessing health care interventions in terms of the value they confer, reports results in terms of the resources that are required for the intervention to produce an additional unit of change in health effectiveness; more economically efficient programs are those with lower cost-effectiveness ratios. This article reviews the methods and results of cost-effectiveness studies in the United States and articulates why routine, voluntary HIV testing is not only of crucial public health importance but also economically justified.
Since 1993, Centers for Disease Control and Prevention (CDC) guidelines have recommended routine, voluntary HIV counseling, testing, and referral for all patients in hospitals where the prevalence of HIV infection is ⩾1% and in settings serving populations known to be at increased risk for HIV infection [1]. These guidelines were revised but largely unchanged in 2001 [2]. The 2001 guidelines included no discussion of the cost or cost-effectiveness of implementing the recommendations, but in fact there had been no economic evaluations of HIV testing published since the antiretroviral therapy (ART) era began [3, 4]. As the AIDS epidemic has matured in the United States, the incidence rate of HIV infection has remained stable, with ~40,000 new infections reported yearly since 1992 [5]. Despite great clinical progress in the treatment of HIV infection, patients are still being identified far too late in the disease process: 40% of patients develop AIDS ≤1 year after learning that they are infected with HIV [6]. Only with earlier case identification and linkage to medical care will patients have the opportunity for timely receipt of ART, which can decrease the morbidity and mortality caused by the disease [7]. To promote the identification and treatment of HIV-infected individuals, the CDC revised its HIV testing guidelines in September 2006, recommending that all persons aged 13–64 years be tested for HIV infection as a matter of routine care—without regard to risk factors—in all health care settings in the United States; annual testing was recommended for persons known to be at high risk for HIV infection [8]. These recommendations have generated both enthusiasm and debate. One notable concern stems from the cost required to embark on testing efforts of this magnitude. Is routine HIV testing worth the cost? Is it cost-effective?
METHODS OF COST-EFFECTIVENESS ANALYSIS
Cost-effectiveness analysis is conducted according to well-established methods that have been described by national panels and journal editors [9–12]. A program is cost-effective if the additional resources needed to implement it result in health gains that are sufficient to justify using the resources in that program. Asserting that a health care screening or treatment program is cost-effective does not imply that it is cost saving nor does it mean that it is simply medically effective.
Cost-effectiveness ratio
In cost-effectiveness analysis, the measure of value is the incremental cost-effectiveness ratio. The numerator of the ratio, the incremental cost, is the difference between the cost of a primary program (i.e., program A) and the cost of an alternative program (i.e., program B). The denominator, the incremental health effectiveness, is a measurement of the health improvement associated with program A relative to that associated with program B. Program B may denote a less intensive variant of program A or the absence of any program. The cost-effectiveness of program A relative to program B is expressed as the following ratio: [cost of program A – cost of program B]/[health improvement produced by program A – health improvement produced by program B]. Because cost-effectiveness ratios are expressed in terms of resource use per unit of benefit conferred, programs that are more economically efficient have lower cost-effectiveness ratios.
Quality-adjusted life-years (QALYs)
Incremental cost can readily be measured in dollars, but to compare programs having diverse health consequences, the health outcomes must be measured in broadly applicable units. In cost-effectiveness analysis, health outcomes are most commonly measured in terms that capture not only increases in survival but also improvements in clinical health that are weighted according to a preference-based measure of well-being. The QALY is one such outcome unit [9].
A QALY is a measure of time spent in a given state of health that would be preferentially equal to 1 year of life spent in perfect health. To obtain the quality-adjusted life expectancy, each possible health state is assigned a numerical weight between 0 (representing a health state equivalent to death) and 1 (denoting perfect health). The scored weights of each health state are subjective and may be based on community surveys of health-state preferences (for policy decisions) or on patient preferences (for individual clinical decisions). As most cost-effectiveness analyses are conducted for the purposes of societal resource allocation, quality-adjustment scores are generally intended to reflect community preferences [9]. The expected duration of time spent in each possible health state is determined, often by means of a mathematical model to extrapolate durations reported in clinical studies with short-term end points. Quality-adjusted life expectancy (measured in QALYs) is obtained by multiplying each health state’s quality weight by the duration of time spent in that health state and then summing over all of the health states. For example, a patient with an expected survival of 8 years, with 4 years of perfect health (utility = 1.0), 1 year in the hospital (utility = 0.5), and 3 years in a nursing home (utility = 0.6), would have a quality-adjusted life expectancy of 6.3 QALYs (i.e., [4 × 1.0] + [1 × 0.5] + [3 × 0.6]).
Discounting
Discounting, or adjusting for the timing of costs and health outcomes, is used in economic analysis to account for the time-specific value of costs and benefits. In general, people prefer to receive health gains sooner and to pay costs later. Discounting quantitatively incorporates these preferences into economic analyses by weighing costs and health outcomes less heavily the further into the future they occur; thus, a health gain in the future is weighted less than an equal immediate gain, and a cost in the future does not weigh as heavily as an immediate cost. As recommended by the Panel on Cost-Effectiveness in Health and Medicine, both the numerator (i.e., costs) and the denominator (i.e., effectiveness) should be discounted at the same rate (generally 3% per year) in all cost-effectiveness analyses [9]. When making health economic decisions for expenditures to be spent over the next 10 years, the question to ask is, what is the present value of all of those expenditures today? For example, at a 3% discount rate, a decision maker would consider $100,000 in 10 years as equivalent to $74,400 today [9]. Discounting is necessary even after all costs have been corrected for price inflation. Before discounting, all costs must be expressed in a single unit of currency at a point in time (e.g., 2006 US dollars).
COST-EFFECTIVENESS OF HIV TESTING
Several interventions in HIV treatment have been analyzed for cost-effectiveness (table 1). Routine HIV testing in the inpatient setting ($38,600 per QALY gained) and for high-risk patients in the outpatient setting every 5 years ($50,000 per QALY gained), although effective, is less cost-effective than several interventions routinely recommended as the standard of HIV clinical care in the United States. These include prophylaxis for Pneumocystis jirovecii pneumonia (PCP) ($2,800 per QALY gained), treatment with ART ($11,700 per QALY gained), and genotype testing after initiation of the first ART regimen ($17,900 per QALY gained) [13–16, 18]. However, routine HIV testing is as or more cost-effective than use of enfuvirtide as salvage ART ($69,500 per QALY gained), prophylaxis against cytomegalovirus infection ($59,000 per QALY gained), or prophylaxis against fungal infection ($240,000 per QALY gained) [19–21].
Table 1.
Cost-effectiveness ratios for treatment of HIV infection.
| Intervention | Treatment(s) | Cost-effectiveness, US$ per QALY gained | Reference |
|---|---|---|---|
| PCP-toxoplasmosis prophylaxis | TMP-SMX | 2,800 | [13] |
| Antiretroviral therapy | AZT, 3TC, EFV | 11,700 | [14] |
| Resistance testing | … | 17,900 | [15] |
| Inpatient HIV testing | … | 38,600 | [16] |
| MAC prophylaxis | AZM | 43,300 | [17] |
| HIV testing of high-risk outpatients every 5 years | … | 50,000 | [18] |
NOTE. AZM, azithromycin; AZT, zidovudine; EFV, efavirenz; MAC, Mycobacterium avium complex; PCP, Pneumocystis jirovecii pneumonia; QALY, quality-adjusted life-year; TMP-SMX, trimethoprim-sulfamethoxazole; 3TC, lamivudine.
The cost-effectiveness of HIV testing compares favorably with many recommended routine screening programs in the United States (table 2). For example, routine HIV testing in the inpatient setting and routine HIV testing of high-risk individuals every 5 years in the outpatient setting are both more cost-effective than recommended routine screening for breast cancer ($57,500 per QALY gained), colon cancer ($57,700 per QALY gained), and type 2 diabetes ($70,000 per QALY gained). In addition, routine HIV testing becomes even more favorable when potential decreases in HIV transmission resulting from the HIV testing program are taken into account [16, 18, 22–26].
Table 2.
Cost-effectiveness of various screening programs in the United States.
| Condition, intervention | Patient age,years | Cost-effectiveness ratio,US$ per QALY gained | Reference |
|---|---|---|---|
| HIV infection | |||
| Testing of inpatients at presentation | Any | 38,600 | [16] |
| Testing of high-risk outpatients every 5 years | Any | 50,000 | [18] |
| Breast cancer, mammography every year | 50–69 | 57,500 | [22] |
| Colon cancer, FOBT and sigmoidoscopy every 5 years | 50–85 | 57,700 | [23] |
| Diabetes mellitus type 2, one-time opportunistic screening of fasting plasma glucose level | > 25 | 70,000 | [24] |
NOTE. FOBT, fecal occult blood test; QALY, quality-adjusted life-year.
Cost-effectiveness of testing in the outpatient setting
In February 2005, the New England Journal of Medicine published 2 reports on the cost-effectiveness of routine HIV testing [18, 25]. Although each study used an independently developed mathematical model that simulated the process of HIV testing and disease progression, as well as independently derived data for model inputs, the results were remarkably similar. Sanders et al. [25] reported that routine testing in a population with a prevalence of undiagnosed HIV infection of 1% produced a cost-effectiveness ratio of $41,000 per QALY gained; the inclusion of transmission benefits decreased the ratio to $15,000 per QALY gained [25]. In the study by Paltiel et al. [18], the cost-effectiveness ratio of routine HIV testing among outpatients was $36,000 per QALY gained for a 3% prevalence of undiagnosed HIV infection, $38,000 per QALY gained for a 1% prevalence, and $113,000 per QALY gained for a 0.1% prevalence [18]. Paltiel and colleagues recently updated this analysis to include the potential transmission effects of a routine HIV testing program [26]. Routine HIV testing remained cost-effective (less than $50,000 per QALY gained) for a prevalence of undiagnosed HIV infection as low as 0.2%. The results of these 3 studies provide strong economic support for the 2006 CDC guidelines calling for routine HIV testing in the outpatient setting [8].
Optimal frequency of retesting
The challenges of examining the impact of routine HIV testing are compounded when one tries to address the optimal frequency of HIV testing. The best strategy for retesting depends on the incidence of HIV infection, which is difficult to determine. Such a strategy also depends on the likelihood that individuals whose HIV infection was not detected during an initial test will be detected on a subsequent test. This is driven by factors such as rates of consent to be tested, rates of receipt of test results, and rates of linkage to health care. Paltiel et al. [18] examined a range of retesting frequencies and noted that, in high-risk populations (defined as a population in which the annual incidence of HIV infection is 1.2%), testing every 5 years remained cost-effective at a ratio of $50,000 per QALY gained; the ratio for annual testing was $100,000 per QALY gained. In lower-risk populations with annual incidence rates ranging from 0.12% to 0.01%, the cost-effectiveness ratio of HIV testing every 5 years ranged from $71,000 to $169,000 per QALY gained and for annual testing ranged from $165,000 to $1,264,000 per QALY gained. In the absence of concrete data on the annual incidence of HIV infection, the current CDC testing guidelines recommend annual screening for persons presumed to be at high risk for HIV infection, including injection drug users, men who have sex with men, persons who exchange sex for money, and the sex partners of these people [8].
Cost-effectiveness of testing in the inpatient setting
Because HIV disease is relatively asymptomatic for many years, infection in many infected individuals is only diagnosed after admission to an inpatient setting for treatment of a new opportunistic infection. A recent model-based analysis of inpatient HIV testing demonstrated that, without routine testing, a majority (53%) of newly diagnosed HIV infections are detected during presentation for an opportunistic infection; this is consistent with data from the CDC [16, 27]. The inclusion of routine testing among inpatients reduced the percentage of HIV infections identified with a new opportunistic infection from 53% to 36%.
As policy makers consider the costs of expanded routine HIV testing programs, the following point should be made clear: what determines the cost-effectiveness of any HIV testing program is not the cost of the HIV test itself—which, by nearly all standards, is inexpensive ($1–$100)—but rather the downstream (i.e., future) costs of HIV care for patients who are identified and then treated for their HIV infection (figure 1). The cost-effectiveness of routine inpatient HIV testing for a population in which the prevalence of HIV infection is 0.1%, ranges only from $38,600 to $86,800 per QALY gained, depending on the cost of the test. As the prevalence of HIV infection increases, the cost of the test becomes less critical; regardless of the test cost, the cost-effectiveness ratio of HIV testing cannot decrease to less than $36,000 per QALY gained. Routine testing will identify more individuals in settings where the prevalence of HIV infection is higher and should, therefore, be more cost-effective. However, the cost-effectiveness of screening cannot become more favorable than $36,000 per QALY gained, because this is now the cost-effectiveness ratio for state-of-the-art HIV care in the United States [16]. This care includes antiretroviral therapy, opportunistic infection prophylaxis and treatment, outpatient care, inpatient care, and laboratory expenditures. It is therefore the care of identified HIV-infected patients that determines the cost, the survival, and the cost-effectiveness of a routine HIV testing program.
Figure 1.
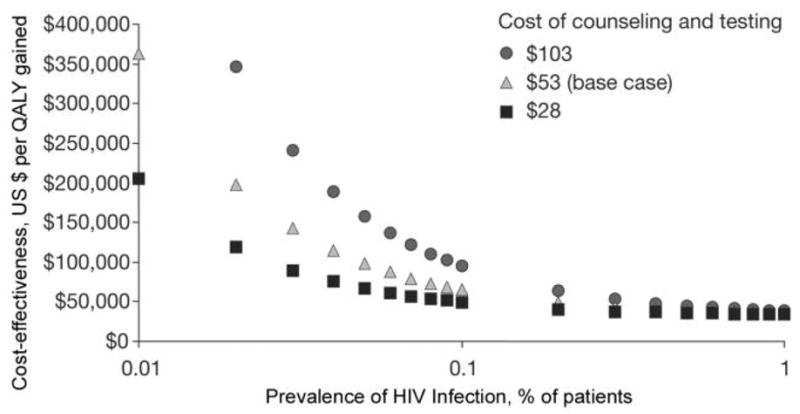
Sensitivity analysis examining the cost of HIV counseling and testing. When the prevalence of HIV infection is > 1%, the lines reflecting cost-effectiveness ratios converge. Only at prevalences of < 0.1% do the costs of counseling and testing drive the cost-effectiveness ratio. QALY, quality-adjusted life-year. Adapted from the following article with permission from Elsevier: Walensky RP, Weinstein MC, Kimmel AD, et al. Routine human immunodeficiency virus testing: an economic evaluation of current guidelines. Am J Med 2005; 118:292–300.
IMPORTANCE OF LINKAGE TO CARE FOR HIV-INFECTED PERSONS
Most discussions of HIV testing, both in the guidelines and in the literature, focus on the need for increased HIV testing, with little discussion of the value of linkage to medical care. Several studies of HIV testing document the challenges of linking infected patients to clinical care [28, 29]. The process of HIV testing should be viewed as a sequential pathway composed of at least 4 important steps: a health care professional offers the test, the patient agrees to undergo the test, the patient receives the test results, and the patient, if infected, is successfully linked to an appropriate care site. Failure of any one of these processes results in the overall failure of the testing system. If the objective is to maximize health benefits, the goal of the testing process lies not only in the detection of HIV-infected individuals but also in the linkage of HIV-infected patients to health care professionals who can care for them.
Walensky et al. [30] attempted to dissect the HIV testing process into these individual steps. In an examination of 100 possible combinations of test acceptance and linkage rates, they found that pathways in which the same number of people were linked to medical care were not equally cost-effective. For example, a program in which 100% of patients were offered an HIV test, 100% took the test, 100% received their test results, and 30% who had positive test results were linked to medical care was less cost-effective than a program in which 100% of patients were offered the test, 30% took the test, 100% who were tested received their test results, and 100% who had positive test results were linked to care. The analysis demonstrated that, other things being equal, programs in which the rate of linkage to medical care is higher than the rate of test acceptance are more cost-effective. These results suggest that if HIV testing dollars are limited, funds should be devoted to ensuring that persons who test positive for HIV infection are effectively linked to care, before they are allocated toward initiating new testing efforts [30].
Lifetime cost of treatment for HIV infection
The cost and cost-effectiveness of routine HIV testing programs are heavily dependent on the cost of treating HIV-infected individuals. In a model-based analysis, Schackman et al. [31] recently projected the lifetime cost of current treatment for HIV infection in the United States. Model input data were derived from the HIV Research Network, a consortium of high-volume primary care sites specializing in the treatment of HIV infection [32, 33]. The analysis focused on a cohort of HIV-infected individuals with a mean CD4 cell count of 310 cells/mm3 and projected a mean survival of 24.2 years after cohort initiation. The discounted lifetime cost of comprehensive treatment per HIV-infected person was $385,000 for this survival benefit (undiscounted cost, $618,900). Antiretroviral therapy accounted for 73% of the total cost, inpatient care accounted for 13%, and outpatient care accounted for 9%.
Although the study by Schackman and colleagues included only the direct medical costs associated with treating HIV infection, another recent study included the cost of lost productivity [34]. Hutchinson et al. [34] projected the total cost associated with the estimated 40,000 new HIV infections expected to occur in the United States every year. The undiscounted total lifetime cost of illness was approximately $53 billion. Medical care accounted for only $8 billion of the total, whereas lost productivity accounted for the remaining $45 billion.
Years of life saved by comprehensive HIV car
Although these costs are considerable, one cannot examine the costs of comprehensive medical care for HIV infection without examining the improvements in health and survival that these expenditures have produced. A recent analysis by Walensky et al. [35] found that > 3 million years of life have been saved in the United States as a direct result of treatment of AIDS. In this analysis, they examined increases in per-person survival since 1989 and the number of people for whom HIV infection was diagnosed, the number who were receiving care, and the number eligible to benefit from increased survival (table 3). Advances in AIDS care were divided into 6 eras, including the pre-ART era, when only PCP and Mycobacterium avium complex prophylaxis were available, and 4 eras of ART. They also included increases in life expectancy associated with the prevention of mother-to-child transmission. For each era, per-person survival benefits for individuals receiving treatment for AIDS were compared with those for individuals whose disease went untreated. Per-person survival benefits ranged from 3.1 months during the era of PCP prophylaxis (in 1989) to 159.9 months during the era of comprehensive care and antiretroviral therapy (in 2003). With initiation of PCP prophylaxis in 1989, median survival for patients with AIDS and a mean CD4 cell count of 87 cells/mm3 was 1.7 years after cohort initiation; in 1996, receipt of first-generation ART boosted median survival to 7.4 years; and by 2003, receipt of fourth-generation ART led to a median per-person survival of 14.1 years. The study noted that these per-person survival gains are superior to those seen for non–small cell lung cancer, node-positive breast cancer, 3-vessel coronary artery disease, and relapsed non-Hodgkin’s lymphoma, as documented over the same period [35].
Table 3.
Per-person survival benefits, numbers of patients with AIDS entering care, and era-specific and cumulative survival benefits.
| Year | Intervention | Mean survival benefit per persona | Patients with AIDS entering care, no. | Patients surviving to next treatment era, % | Infections averted, no. | Total survival benefit, years |
|---|---|---|---|---|---|---|
| HIV disease treatment | ||||||
| 1989–1992 | PCP prophylaxis | 3.1 months | 158,370 | 33 | … | 40,912 |
| 1993–1995 | PCP/MAC prophylaxis | 24.4 months | 226,458 | 39 | … | 460,465 |
| 1996–1997 | PCP/MAC prophylaxis and ART 1 | 93.7 months | 72,716 | 86 | … | 567,788 |
| 1998–1999 | PCP/MAC prophylaxis and ART 2 | 132.6 months | 52,702 | 93 | … | 582,359 |
| 2000–2002 | PCP/MAC prophylaxis and ART 3 | 138.8 months | 71,946 | 91 | … | 832,179 |
| 2003 | PCP/MAC prophylaxis and ART 4 | 159.9 months | 24,780 | … | … | 330,189 |
| Subtotal | 2,813,892 | |||||
| PMTCT | ||||||
| 1994–1999 (pMTCT-ZDV) | Child receives OI prophylaxis and ZDV monotherapy | 60.5 years | … | … | 223 | 51,646 |
| 1994–1999 (pMTCT-ZDV) | Child receives OI prophylaxis and combination ART | 45.8 years | … | … | 833 | … |
| 2000–2003 (pMTCT-cART) | Child receives OI prophylaxis and combination ART | 46.7 years | … | … | 1839 | 85,833 |
| Subtotal | 137,479 | |||||
| Total | 2,951,371 | |||||
NOTE. cART, combination antiretroviral therapy; MAC, Mycobacterium avium complex; OI, opportunistic infection; PCP, Pneumocystis jirovecii; pMTCT, prevention of mother-to-child transmission; ZDV, zidovudine. Adapted from [35].
Data are reported as weighted averages of the per-person survival benefit among individuals receiving care, derived from each year in the current treatment era, and reflect changes in both the life expectancy of HIV-positive patients and the general US population over time.
CONCLUSIONS
Survival gains among patients with HIV disease in the United States have increased dramatically over time. The full extent of these increases, however, is achievable only for patients who are identified with HIV infection and receive appropriate care. This review has summarized substantial data from the literature that document that routine, voluntary HIV testing, as recommended in the 2006 CDC guidelines, is a cost-effective intervention. HIV testing is relatively inexpensive and highly effective, and diagnosis of HIV infection can, when followed by prompt linkage to medical care, lead to a sequence of events, including initiation of ART, that yield substantial survival benefits. For this reason, efforts to implement routine HIV testing must be accompanied by a simultaneous clinical and public health commitment to link HIV-infected persons to medical care and to ensure that adequate funding for treatment of HIV infection is available.
Acknowledgments
We thank Neil Canavan, for assistance in preparing this manuscript, and Mariam Fofana, for technical assistance.
The “Opportunities for Improving HIV Diagnosis, Prevention & Access to Care in the U.S.” conference was sponsored by the American Academy of HIV Medicine, amfAR, the Centers for Disease Control and Prevention, the Forum for Collaborative HIV Research, the HIV Medicine Association of the Infectious Diseases Society of America, and the National Institute of Allergy and Infectious Diseases. Funding for the conference was supplied through an unrestricted educational grant from Gilead Sciences, amfAR, GlaxoSmithKline, Pfizer, Abbott Virology, OraSure Technologies, Roche Diagnostics, and Trinity Biotech.
Financial support. The National Institute of Allergy and Infectious Diseases (R01 AI42006, K24 AI062476, and P30 AI060354), the National Institute of Mental Health (R01 MH65869 and R01 MH073445), the National Institute on Drug Abuse (R01 DA015612), and the Doris Duke Charitable Foundation (Clinical Scientist Development Award).
Footnotes
Presented in part: Opportunities for Improving HIV Diagnosis, Prevention & Access to Care in the U.S., Washington, D.C., 29–30 November 2006.
Supplement sponsorship. This article was published as part of a supplement entitled “Opportunities for Improving the Diagnosis of, Prevention of, and Access to Care for HIV Infection in the United States,” sponsored by the American Academy of HIV Medicine, amfAR, the Centers for Disease Control and Prevention, the Forum for Collaborative HIV Research, the HIV Medicine Association of the Infectious Diseases Society of America, and the National Institute of Allergy and Infectious Diseases.
Potential conflicts of interest. All authors: no conflicts.
References
- 1.Centers for Disease Control and Prevention. Recommendations for HIV testing services for inpatients and outpatients in acute-care hospital settings. MMWR Morb Mortal Wkly Rep. 1993;42:157–8. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Revised guidelines for HIV counseling, testing, and referral. MMWR Recomm Rep. 2001;50(RR19):1–57. [PubMed] [Google Scholar]
- 3.Owens DK, Nease RF, Jr, Harris RA. Cost-effectiveness of HIV screening in acute care settings. Arch Intern Med. 1996;156:394–404. [PubMed] [Google Scholar]
- 4.Lurie P, Avins AL, Phillips KA, Kahn JG, Lowe RA, Ciccarone D. The cost-effectiveness of voluntary counseling and testing of hospital in-patients for HIV infection. JAMA. 1994;272:1832–8. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Guidelines for national human immunodeficiency virus case surveillance, including monitoring for human immunodeficiency virus infection and acquired immunodeficiency syndrome. MMWR Recomm Rep. 1999;48:1–28. [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Late versus early testing of HIV--16 sites, United States, 2000–2003. MMWR Morb Mortal Wkly Rep. 2003;52:581–6. [PubMed] [Google Scholar]
- 7.Holmberg SD, Palella FJ, Jr, Lichtenstein KA, Havlir DV. The case for earlier treatment of HIV infection. Clin Infect Dis. 2004;39:1699–704. doi: 10.1086/425743. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Revised recommendation for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR14):1–17. [PubMed] [Google Scholar]
- 9.Gold MR, Siegel JE, Russel LB, Weinstein MC. Cost-effectiveness in health and medicine. New York, NY: Oxford University Press; 1996. [Google Scholar]
- 10.Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ. 1996;313:275–83. doi: 10.1136/bmj.313.7052.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Economic analysis of health care technology: a report on principles. Task Force on Principles for Economic Analysis of Health Care Technology. Ann Intern Med. 1995;123:61–70. doi: 10.7326/0003-4819-123-1-199507010-00011. [DOI] [PubMed] [Google Scholar]
- 12.National Institute for Health and Clinical Excellence. [Accessed 22 March 2007];Guide to the methods of technology appraisal. :201974. Available at: http://www.nice.org.uk/page.aspx?o. [PubMed]
- 13.Goldie SJ, Kaplan JE, Losina E, et al. Prophylaxis for human immunodeficiency virus–related Pneumocystis carinii pneumonia: using simulation modeling to inform clinical guidelines. Arch Intern Med. 2002;162:921–8. doi: 10.1001/archinte.162.8.921. [DOI] [PubMed] [Google Scholar]
- 14.Freedberg KA, Losina E, Weinstein MC, et al. The cost effectiveness of combination antiretroviral therapy for HIV disease. N Engl J Med. 2001;344:824–31. doi: 10.1056/NEJM200103153441108. [DOI] [PubMed] [Google Scholar]
- 15.Weinstein MC, Goldie SJ, Losina E, et al. Use of genotypic resistance testing to guide HIV therapy: clinical impact and cost-effectiveness. Ann Intern Med. 2001;134:440–50. doi: 10.7326/0003-4819-134-6-200103200-00008. [DOI] [PubMed] [Google Scholar]
- 16.Walensky RP, Weinstein MC, Kimmel AD, et al. Routine human immunodeficiency virus testing: an economic evaluation of current guidelines. Am J Med. 2005;118:292–300. doi: 10.1016/j.amjmed.2004.07.055. [DOI] [PubMed] [Google Scholar]
- 17.Freedberg KA, Scharfstein JA, Seage GR, 3rd, et al. The cost-effectiveness of preventing AIDS-related opportunistic infections. JAMA. 1998;279:130–6. doi: 10.1001/jama.279.2.130. [DOI] [PubMed] [Google Scholar]
- 18.Paltiel AD, Weinstein MC, Kimmel AD, et al. Expanded screening for HIV in the United States—an analysis of cost-effectiveness. N Engl J Med. 2005;352:586–95. doi: 10.1056/NEJMsa042088. [DOI] [PubMed] [Google Scholar]
- 19.Sax PE, Losina E, Weinstein MC, et al. Cost-effectiveness of enfuvirtide in treatment-experienced patients with advanced HIV disease. J Acquir Immune Defic Syndr. 2005;39:69–77. doi: 10.1097/01.qai.0000160406.08924.a2. [DOI] [PubMed] [Google Scholar]
- 20.Paltiel AD, Goldie SJ, Losina E, et al. Preevaluation of clinical trial data: the case of preemptive cytomegalovirus therapy in patients with human immunodeficiency virus. Clin Infect Dis. 2001;32:783–93. doi: 10.1086/319223. [DOI] [PubMed] [Google Scholar]
- 21.Scharfstein JA, Paltiel AD, Freedberg KA. The cost-effectiveness of fluconazole prophylaxis against primary systemic fungal infections in AIDS patients. Med Decis Making. 1997;17:373–81. doi: 10.1177/0272989X9701700402. [DOI] [PubMed] [Google Scholar]
- 22.Salzmann P, Kerlikowske K, Phillips K. Cost-effectiveness of extending screening mammography guidelines to include women 40 to 49 years of age. Ann Intern Med. 1997;127:955–65. doi: 10.7326/0003-4819-127-11-199712010-00001. [DOI] [PubMed] [Google Scholar]
- 23.Frazier AL, Colditz GA, Fuchs CS, Kuntz KM. Cost-effectiveness of screening for colorectal cancer in the general population. JAMA. 2000;284:1954–61. doi: 10.1001/jama.284.15.1954. [DOI] [PubMed] [Google Scholar]
- 24.The cost-effectiveness of screening for type 2 diabetes. CDC Diabetes Cost-Effectiveness Study Group, Centers for Disease Control and Prevention. JAMA. 1998;280:1757–63. [PubMed] [Google Scholar]
- 25.Sanders GD, Bayoumi AM, Sundaram V, et al. Cost-effectiveness of screening for HIV in the era of highly active antiretroviral therapy. N Engl J Med. 2005;352:570–85. doi: 10.1056/NEJMsa042657. [DOI] [PubMed] [Google Scholar]
- 26.Paltiel AD, Walensky RP, Schackman BR, et al. Expanded HIV screening in the United States: effect on clinical outcomes, HIV transmission, and costs. Ann Intern Med. 2006;145:797–806. doi: 10.7326/0003-4819-145-11-200612050-00004. [DOI] [PubMed] [Google Scholar]
- 27.Schwarcz S, Hsu L, Dilley JW, Loeb L, Nelson K, Boyd S. Late diagnosis of HIV infection: trends, prevalence, and characteristics of persons whose HIV diagnosis occurred within 12 months of developing AIDS. J Acquir Immune Defic Syndr. 2006;43:491–4. doi: 10.1097/01.qai.0000243114.37035.de. [DOI] [PubMed] [Google Scholar]
- 28.Samet JH, Freedberg KA, Savetsky JB, Sullivan LM, Stein MD. Understanding delay to medical care for HIV infection: the long-term non-presenter. AIDS. 2001;15:77–85. doi: 10.1097/00002030-200101050-00012. [DOI] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention. Routinely recommended HIV testing at an urban urgent-care clinic—Atlanta, Georgia, 2000. MMWR Morb Mortal Wkly Rep. 2001;50:538–41. [PubMed] [Google Scholar]
- 30.Walensky RP, Weinstein MC, Smith HE, Freedberg KA, Paltiel AD. Optimal allocation of testing dollars: the example of HIV counseling, testing, and referral. Med Decis Making. 2005;25:321–9. doi: 10.1177/0272989X05276955. [DOI] [PubMed] [Google Scholar]
- 31.Schackman BR, Gebo KA, Walensky RP, et al. The lifetime cost of current human immunodeficiency virus care in the United States. Med Care. 2006;44:990–7. doi: 10.1097/01.mlr.0000228021.89490.2a. [DOI] [PubMed] [Google Scholar]
- 32.Betz ME, Gebo KA, Barber E, et al. Patterns of diagnoses in hospital admissions in a multistate cohort of HIV-positive adults in 2001. Med Care. 2005;43:III3–14. doi: 10.1097/01.mlr.0000175632.83060.eb. [DOI] [PubMed] [Google Scholar]
- 33.Fleishman JA, Gebo KA, Reilly ED, et al. Hospital and outpatient health services utilization among HIV-infected adults in care 2000–2002. Med Care. 2005;43:III40–52. doi: 10.1097/01.mlr.0000175621.65005.c6. [DOI] [PubMed] [Google Scholar]
- 34.Hutchinson AB, Farnham PG, Dean HD, et al. The economic burden of HIV in the United States in the era of highly active antiretroviral therapy: evidence of continuing racial and ethnic differences. J Acquir Immune Defic Syndr. 2006;43:451–7. doi: 10.1097/01.qai.0000243090.32866.4e. [DOI] [PubMed] [Google Scholar]
- 35.Walensky RP, Paltiel AD, Losina E, et al. The survival benefits of AIDS treatment in the United States. J Infect Dis. 2006;194:11–9. doi: 10.1086/505147. [DOI] [PubMed] [Google Scholar]


