Fig. 2. Purification and activity of HMM II-B0 and II-B2 protein.
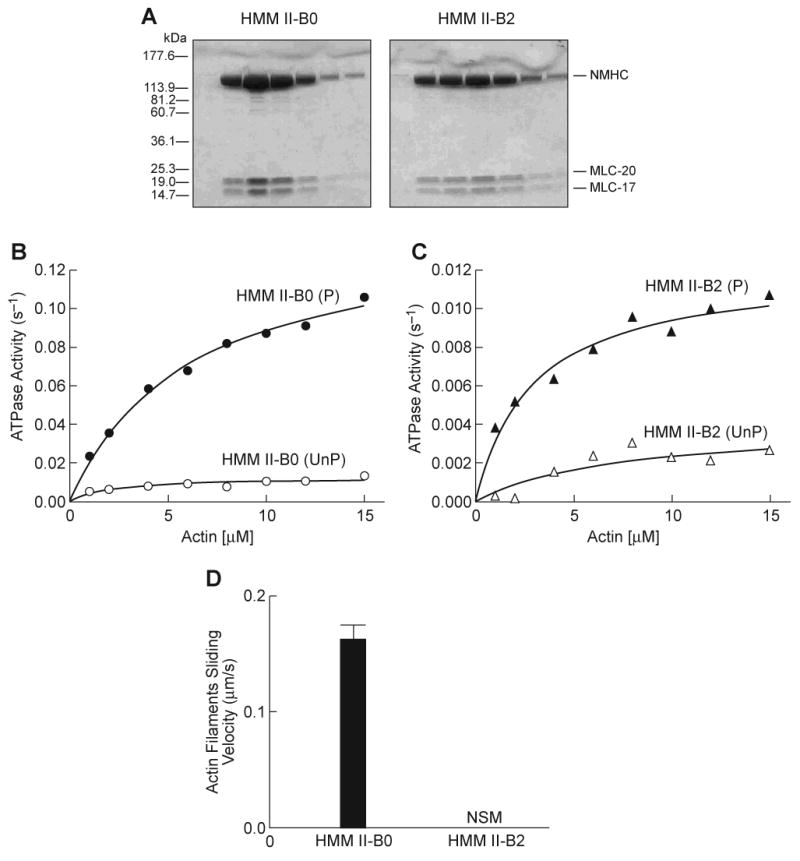
(A) Coomassie Blue-stained SDS-polyacrylamide gels of column fractions of the concentrated HMM II-B0 and II-B2 eluted from a Q-Sepharose column. (B) Actin-activated MgATPase activity of HMM II-B0 was measured before (open circles) and after (closed circles) phosphorylation by MLCK at 25°C. (C) Actin-activated MgATPase activity of HMM II-B2 before (open triangles) and after (closed triangles) phosphorylation by MLCK. The MgATPase activity of HMM in the absence of actin was subtracted from each data point. Datasets were fitted to a hyperbolic equation (solid lines) to determine the kinetic constants, Vmax and KATPase (see Table 1). The data shown are from a single preparation of each HMM. A total of four different preparations were used to prepare Table 1. Note the different scales of ATPase activity (s-1) for panels B and C. UnP, unphosphorylated; P, phosphorylated. (D) In vitro motility rate for actin filaments using HMM II-B0 or II-B2. Each HMM II-B protein was introduced at a concentration of 0.2 mg/ml into the flow chamber. The sliding velocity was determined for three preparations each of HMM II-B0 and II-B2. The plot shows mean velocity with the standard deviation. No significant movement (NSM) of actin filaments was seen with the HMM II-B2 preparation. All proteins were phosphorylated with MLCK prior to the IVM assays.
