Fig. 4. In vitro phosphorylation of HMM II-B2 by Src kinase.
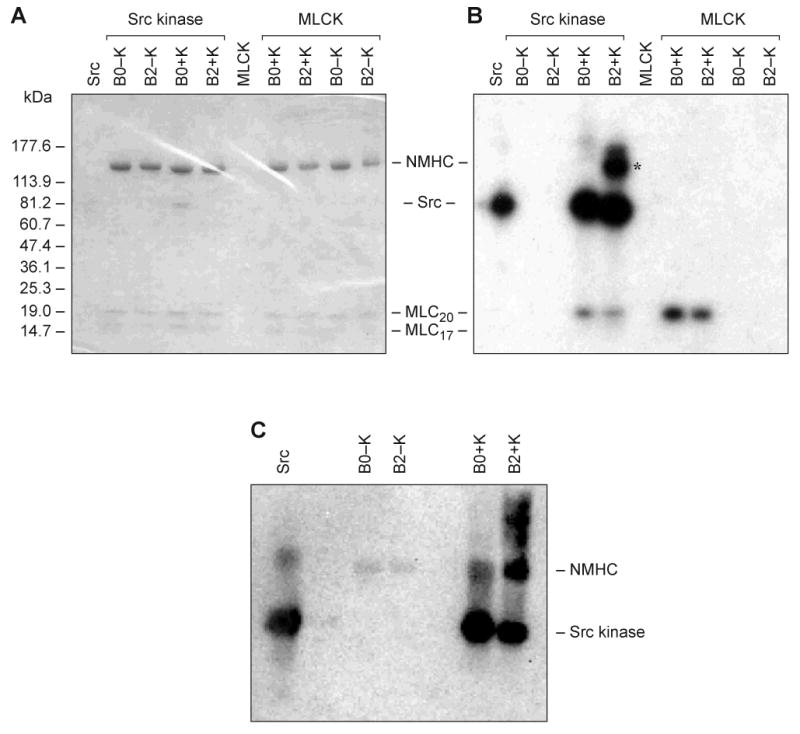
Baculovirus purified HMM II-B0 and II-B2 were incubated with (+K) and without (−K) Src kinase or MLCK. Src kinase alone was incubated without HMM II-B. (A) Coomassie Blue-stained SDS 4-20% polyacrylamide gel of the samples following incubation with the phosphorylation mixture. (B) Autoradiogram of A showing phosphorylation of HMM II-B2 and autophosphorylation of Src kinase. The heavy chain of II-B2 containing the 21-aa insert is phosphorylated (B2+K; Src kinase), but not the II-B0 heavy chain. The asterisk indicates HMM II-B2 heavy chain phosphorylation. The 20 kDa MLC (MLC-20) is also phosphorylated by Src kinase to a small extent. The marker for standard proteins is shown on the left. MLCK only phosphorylated MLC-20. Note that the specific activity of the [γ-32P] ATP was approximately 10X greater for the Src kinase reaction mixtures. (C) Immunoblot analysis of phosphorylated HMM II-B2 after incubation with Src kinase. The blot was incubated with an anti-phosphotyrosine specific antibody.
