FIGURE 4.
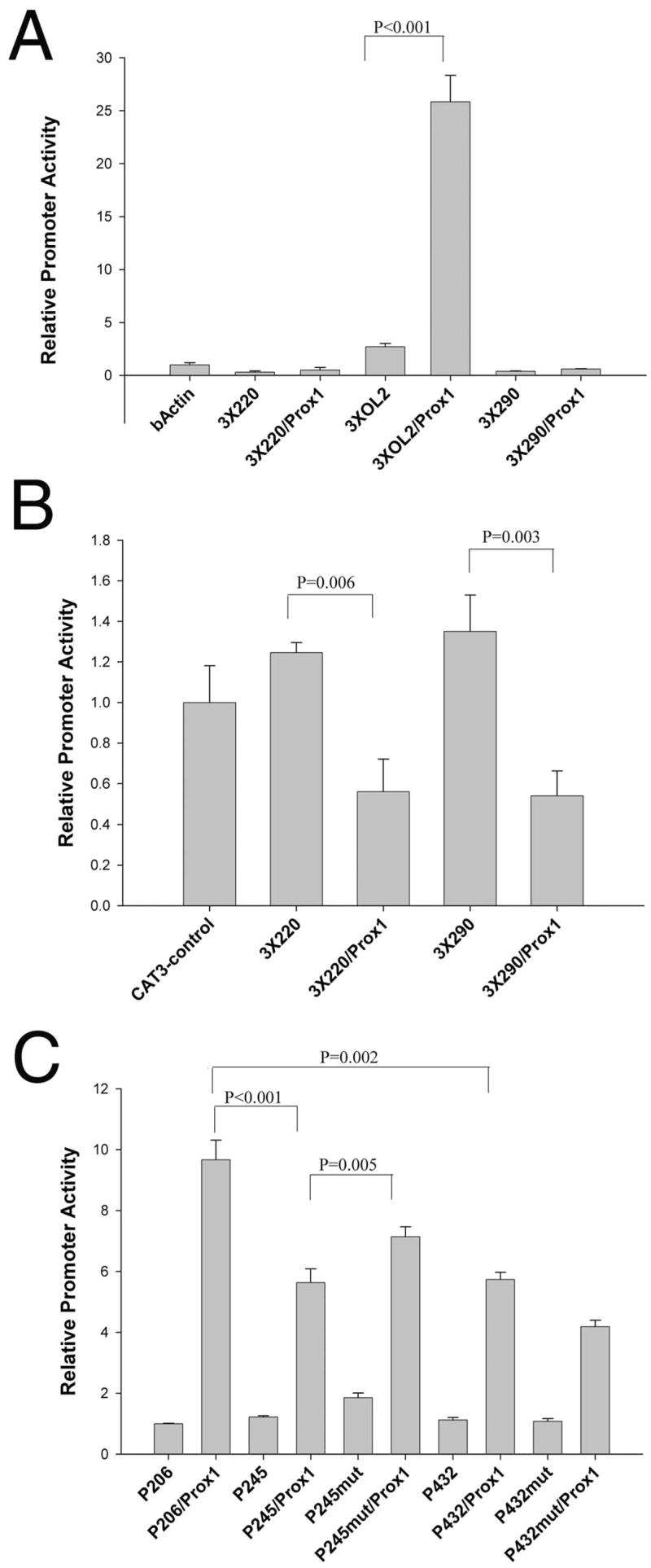
Functional analysis of the −220 and −290 Prox1-binding sites. (A) The ability of the −220, −290, and OL2 elements to confer Prox1 responsiveness to a heterologous promoter. Trimers of each element were placed upstream of the chicken β-actin basal promoter, linked to CAT, and transfected into CHO cells. The OL2 element was able to confer Prox1 responsiveness on this promoter (P ≤ 0.001), but neither −220 nor −290 had a similar effect. (B) The ability of −220 and −290 to confer Prox1-mediated repression on a heterologous promoter. Trimers of the −220 and −290 element were ligated up-stream of the SV40 promoter of the pCAT3-control plasmid, which also contained a strong enhancer. Neither the −220 nor the −290 elements alone significantly affected the activity of the vector in CHO cell transfections (P = 0.06). However, cotransfection with a Prox1 expression vector led to 40% reduction in the activity of 3 × 220 and 3 × 290 pCAT control that was statistically significant (P = 0.006). (C) Prox1 responsiveness of the upstream portions of the βB1-crystallin promoter. The promoter −206/+30 had the most robust response to Prox1 cotransfection, whereas the inclusion of sequences from −207 to −245, which encompassed the −220 Prox1 element, significantly reduced Prox1 responsiveness of the promoter. Mutation of the −220 element in the context of the −245/+30 fragment significantly increased Prox1 responsiveness of this fragment (P < 0.005). The −432/+30 fragment containing all three known Prox1-binding sites was also significantly less Prox1 responsive than −206/+30, which only contained the OL2 site (P = 0.002); however, its Prox1 responsiveness was not significantly different from −245/+30. Notably, mutation of the −220 element in the context of the −432/+30 promoter reduced Prox1 responsiveness of this fragment.
