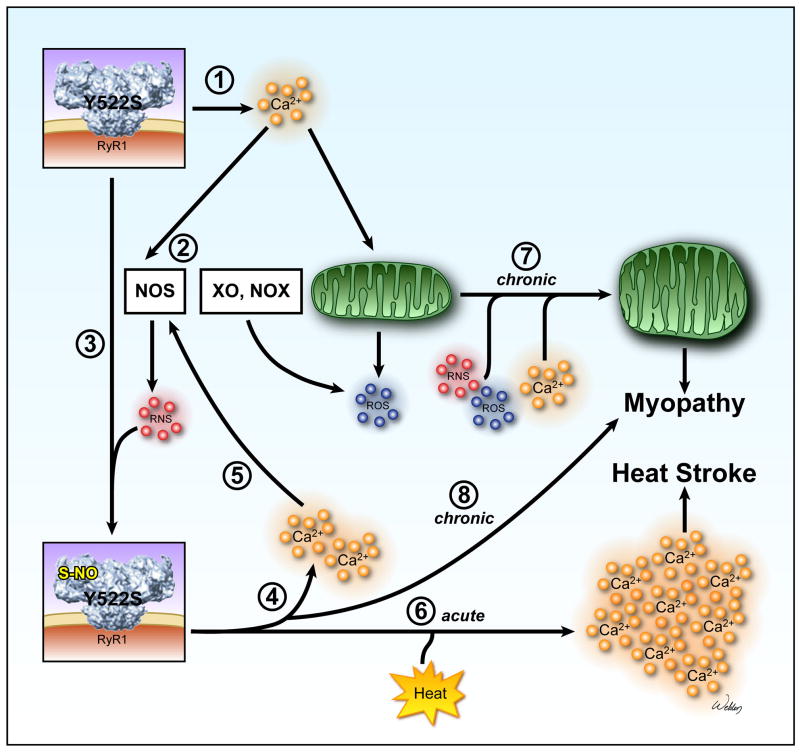
Figure 5. Proposed model of exertional/environmental heat stress and myopathy in RyR1Y522S/wt mice.
➀ SR Ca2+ release channels in RyR1Y522S/wt mice are more sensitive to voltage, ligand, and Ca2+ activation and open more readily, producing small, possibly local, increases in resting Ca2+. ➁ Increased cytosolic Ca2+ levels enhance ROS/RNS production. Increases in RNS are likely to be produced by nitric oxide synthases (NOS). Possible sources of ROS include mitochondria, xanthine oxidase (XO) and NAD(P)H oxidases (NOX). ➂ Although both S-glutathionylation and S-nitrosylation of RyR1Y522S/wt occurs, our data suggest that S-nitrosylation alone enhances the temperature sensitivity of RyR1. ➃ S-nitrosylation of RyR1Y522S/wt increases its sensitivity to temperature and decreases its sensitivity to Ca2+ inhibition further promoting SR Ca2+ leak. ➄ Ca2+ increases further enhance ROS/RNS production. ➅ In response to heat stress, Ca2+ release from the modified RyR1Y522S/wt is greatly and persistently augmented, leading to heat stroke. ➆ and ➇ Chronically-elevated levels of Ca2+ and ROS/RNS damage mitochondria and contribute to the development of myopathy.