Fig. 1.
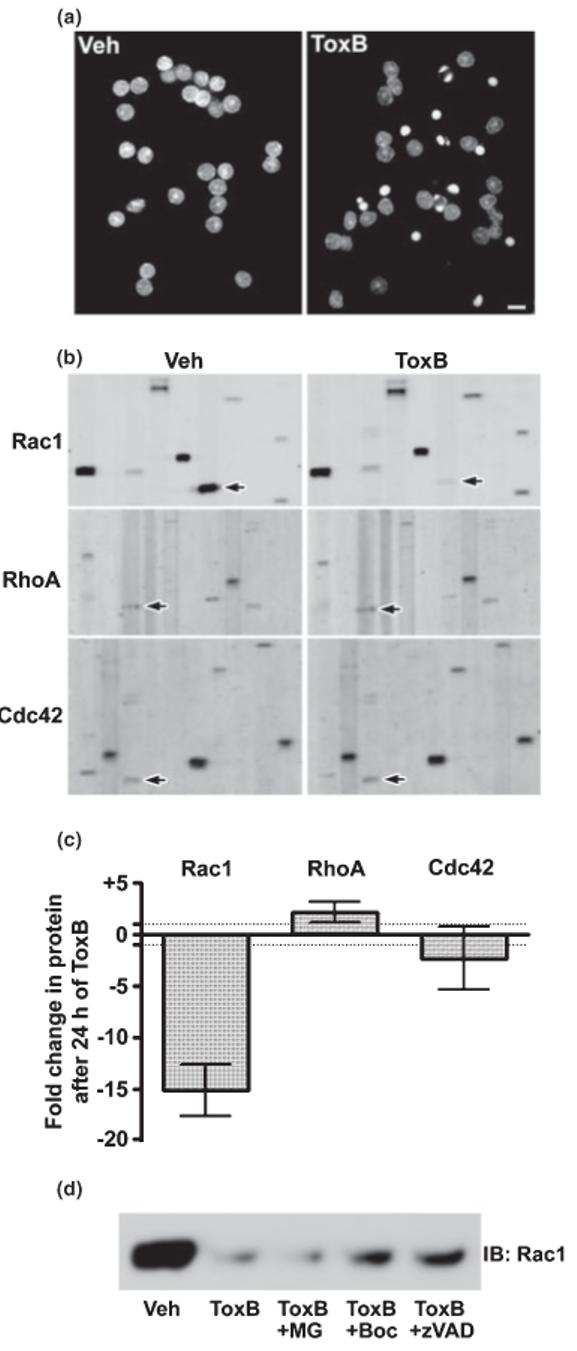
ToxB induces apoptosis and elicits a selective caspase-mediated degradation of Rac1 GTPase in CGNs. (a) CGNs were incubated for 24 h in complete medium (containing serum and 25 mm KCl) in the presence of either vehicle (Veh; 2 μg/mL BSA in PBS) or ToxB (40 ng/mL). Following incubation, cells were fixed in paraformaldehyde and nuclei were stained with Hoechst dye. CGNs exposed to ToxB showed marked nuclear condensation and fragmentation characteristic of apoptosis. Scale bar, 10 μm. (b) Representative immunoblots of lysates obtained from CGNs incubated as described in (a). The blots shown were obtained from the BD PowerBlot™ (see Experimental procedures for details) using specific monoclonal antibodies against Rac1 (upper blots), RhoA (middle blots), or Cdc42 (lower blots). The immunoreactive Rho family GTPases are indicated by the arrows. Other protein bands apparent on the membranes represent adjacent lanes of the PowerBlot™ that were probed for distinct proteins and are shown to give an indication of the overall equality of protein loading. (c) The fold change in protein content induced by 24 h of ToxB treatment for each of the Rho family members blotted for in (b) is shown. Data represent the means ± range of duplicate PowerBlot™ comparisons for each GTPase. The dotted lines indicate no change (-1 to +1 fold). (d) CGNs were exposed for 8 h to ToxB in the absence or presence of either a pan-caspase inhibitor (Boc-Asp(OMe)-FMK; 200 μm) or zVAD-FMK; 200 μm) or a proteasome inhibitor (MG132; 10 μm). Following incubation, cell lysates were immunoblotted for Rac1.
