Fig. 3.
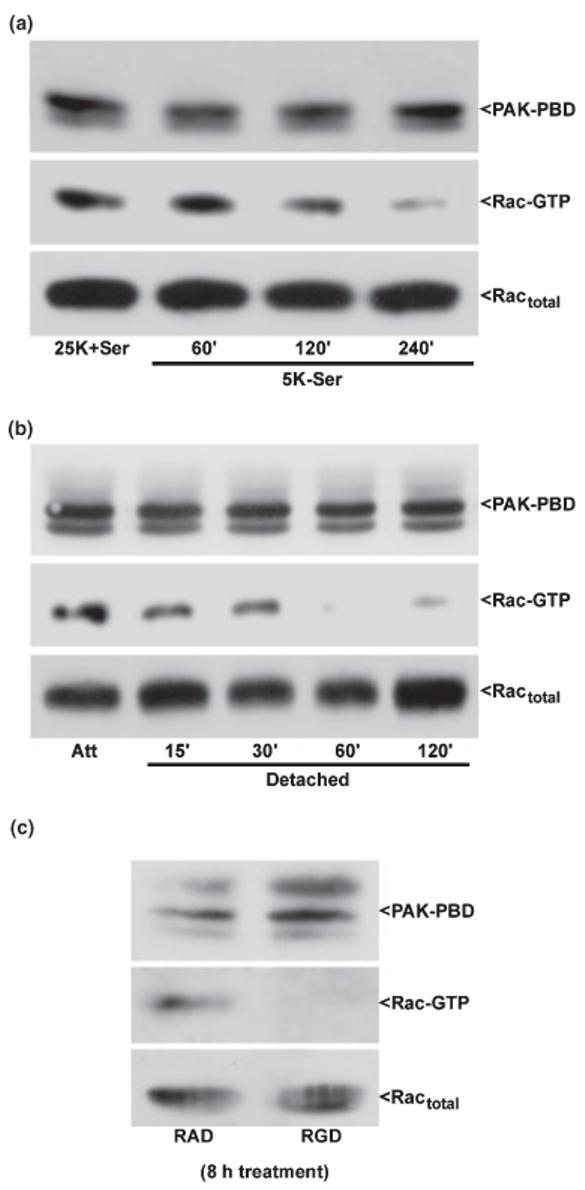
Rac1 GTP-loading is maintained in CGNs by trophic support and integrin-mediated cell attachment. CGNs were incubated under either attached conditions (a) in trophic factor-deprived medium containing 5 mm KCl and lacking serum (5K-Ser) for up to 4 h, detached conditions (b) for up to 2 h in medium containing 25 mm KCl and serum (25K + Ser), or incubated with RAD or RGD peptides (c) for 8 h. Following incubation, CGNs were immediately lysed on ice into a high Mg2+ buffer. Cell debris was removed by brief centrifugation and a small aliquot of the resulting lysate was separated for quantitation of total Rac in each sample. The remaining cell lysates were incubated for 1 h with an excess of the protein binding domain of the Rac effector, PAK (PAK-PBD), bound to agarose beads. The pellet was washed three times with lysis buffer and samples were electrophoresed through 15% polyacrylamide gels. Proteins were transferred to PVDF membranes which were immunoblotted with a monoclonal antibody against Rac1 and an HRP-conjugated secondary antibody. Immunoreactive Rac1 protein was detected using standard ECL techniques. GTP-loaded Rac was brought down in the PAK-PBD precipitation (middle blots). The PAK-PBD is detected non-specifically by the secondary antibody and is shown as a control for equal loading (upper blots). The total Rac in each lysate is also shown as a loading control as no significant loss is detected in Rac protein under these conditions (bottom blots). The blots shown are representative of results obtained in three separate experiments. Att, attached control.
