Abstract
Multiple cofactors and chromatin remodeling complexes have been identified to contribute to the transcriptional activation regulated by thyroid hormone receptors (TRs) in vitro. However, their role and function during development in vivo remains to be elucidated. The total dependence of amphibian metamorphosis on thyroid hormone (T3) provides a unique vertebrate model for studying the molecular mechanism of TR function in vivo. In this study, we show that the expression of Brahma-related gene 1 (BRG1), a chromatin-remodeling enzyme, is up-regulated at the climax of Xenopus laevis metamorphosis, whereas BRG1-associated factor 57 (BAF57), a BRG1-binding protein in BRG1-containing chromatin remodeling complexes, is constitutively expressed during development. Consistently, T3 treatment of premetamorphic tadpoles led to up-regulation of the expression of BRG1 but not BAF57. Studies using a reconstituted T3-dependent Xenopus oocyte transcription system, where we could study TR function in the context of chromatin, revealed that BRG1 enhances the transcriptional activation by ligand-bound TRs in a dose-dependent manner, whereas a remodeling-defective BRG1 mutant inhibited the activation, suggesting that this process relies on chromatin remodeling. Additional studies showed that BAF57 interacted with BRG1 in oocytes and enhanced gene activation by TR cooperatively with BRG1 in vivo. Chromatin immunoprecipitation revealed that BAF57 was recruited to the TR-regulated promoter in the presence of TR and T3. Together, these findings suggest a role of BRG1/BAF57-containing chromatin remodeling complexes in TR-regulated gene expression during postembryonic development.
THE THYROID HORMONE (T3) receptor (TR), a member of the nuclear hormone receptor superfamily, is a transcription factor regulated by the interaction with its ligand, T3. TRs play an essential role in animal development, homeostasis, and pathogenesis (1,2,3,4,5,6,7,8). TR regulates transcription by forming a heterodimer with 9-cis-retinoic acid receptor (RXR), another nuclear receptor, and binds to T3 response elements (TREs) in the proximity of the promoters of target genes (2,3,4,9,10). This dimerization occurs independently of ligand interaction, but transcriptional regulation by TR is determined by T3. In the absence of ligand, TRs exert repressive effects on promoters in the context of chromatin via the recruitment of corepressor complexes. Conversely, in the presence of T3, TRs activate transcription by recruiting coactivator complexes that must overcome the barrier to transcription that represents the tightly packed chromatin fiber and recruit basal transcription factors and RNA polymerase II (10,11,12,13,14). Many different TR coactivators, whose actions are mediated through covalent modification of histone proteins, have been identified to modify the chromatin structure, and their roles in regulating cellular processes such as cell transformation, differentiation, and apoptosis have been extensively studied in vitro (4,15,16,17,18,19,20,21,22,23). In addition to histone-modifying coactivators, multiple ATP-dependent chromatin remodeling complexes have been identified to be important cofactor complexes for transcriptional activation (21,24,25,26,27,28). These different classes of cofactors likely act in a concerted manner and contribute to the generation of a dynamic chromatin structure around the promoters to modulate gene transcription.
Several chromatin remodeling complexes, such as mating type switching/sucrose nonfermenting (SWI/SNF), use energy derived from ATP hydrolysis to disrupt histone-DNA interactions and have been implicated in the regulation of transcriptional activation (26,29,30,31,32,33,34,35,36,37,38,39,40). SWI/SNF was the first chromatin remodeling complex identified to be involved in gene regulation by nuclear hormone receptors (26). It has been suggested to participate in the modification of nucleosomal structure leading to both transcriptional activation and repression. In vitro assays with several nuclear hormone receptors, including the estrogen and glucocorticoid receptors, have shown that SWI/SNF can modify the pattern of nucleosomal positioning and aid local disruption within a preassembled nucleosomal array (26,41,42,43,44). This process has been shown to recruit transcriptional coactivators for RNA polymerase II to proceed through downstream nucleosome-bound promoter. However, the role and function of the SWI/SNF complex in vivo during development remains to be delineated.
SWI/SNF is a class of large, 2-MDa chromatin remodeling complexes. Each vertebrate SWI/SNF complex includes either Brahma-related gene 1 (BRG1) or Brahma (BRM) DNA-dependent ATPase, and their associated factors (BAFs). The role of each BAF has not been completely elucidated, but it has been speculated that the BAF subunits largely contribute to SWI/SNF specificity (45,46,47,48). In vitro studies with human BRG1 and BRM suggest that BAF170, BAF155, and BAF45 are required for reconstituting chromatin remodeling, whereas BAF60 and BAF57 mediates interaction with transcriptional coactivators or corepressors (49,50,51,52).
Of particular interest is the 57-kDa subunit of the SWI/SNF complexes, BAF57. It is present only in higher eukaryotes (47,48,49). BAF57 is present in all mammalian SWI/SNF complexes and contains a high-mobility-group domain adjacent to a kinesin-like region. BAF57 was suggested to play topological roles as the BRG1 complex enters or exits the nucleosome (31). BAF57 has been linked to the regulation of cancer growth, with many studies focusing on its role in breast and prostrate cancers (52,53,54,55,56,57). The BAF57 subunit has been shown to directly interact with the glucocorticoid and androgen receptors and promote their activity (51,55). The BAF57 subunit has also been shown to interact with both the estrogen receptor and the p160/steroid receptor coactivator (SRC) (52,53). Collectively, these observations implicate critical roles for BAF57 in regulating the interaction between SWI/SNF and nuclear hormone receptors. To date, the role of BAF57 and how it may regulate TR transcriptional action during development have not been examined.
To address this issue, the present study used Xenopus laevis as a model system to investigate the interplay of BRG1 and BAF57 in TR function during T3-regulated gene expression and postembryonic development. The total dependence of amphibian metamorphosis on T3 provides a unique vertebrate model for studying the molecular mechanisms of TR function in vivo (3,58). Anuran metamorphosis shares similarities to mammalian postembryonic development. An advantage of using the Xenopus model is that the metamorphosis of tadpoles can be easily induced by the addition of exogenous T3 to the tadpole-rearing water, causing all the morphological changes, organ transformations, and tissue remodeling that are associated with natural metamorphosis. Unlike developing mammalian embryos, tadpoles are free living, and therefore, maternal hormones do not influence their development. More importantly, we and others have shown that TR is not only necessary but also sufficient to mediate the metamorphic effects of T3 (59,60,61,62). All these reasons make amphibian metamorphosis an excellent system to investigate the role of transcriptional cofactors in gene regulation by TR in development. Using this model system, we analyzed the expression of BRG1 and BAF57 during natural and T3-induced metamorphosis. We showed that BRG1 expression was up-regulated during metamorphosis, whereas BAF57 was constitutively expressed, supporting a role for both during amphibian development. In the Xenopus oocyte system, where we can study gene regulation by TR in the context of chromatin, both BRG1 and BAF57 enhanced TR-mediated gene transcription in a dose-dependent manner in the presence of T3. We further demonstrated that BAF57 interacted with BRG1 and was recruited by liganded TRs to its target promoter. Together, these findings suggest a role of BAF57-containing BRG1 complexes in TR-regulated gene expression during animal development.
RESULTS
Differential Expression of BRG1 and BAF57 during Natural Metamorphosis
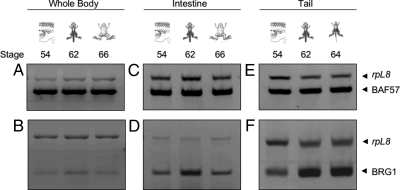
To investigate whether BRG1 and BAF57 play a role in gene regulation by TR during postembryonic development of X. laevis, their expression profiles were initially examined in whole animals at various stages of metamorphosis. Total RNA was isolated from premetamorphic (stage 54), metamorphic (stage 62), and postmetamorphic (stage 66) animals (five for each stage) followed by RT-PCR analysis. The data showed that the expression of BAF57 remained relatively constant throughout metamorphosis (Fig. 1A). In contrast, BRG1 was elevated at stage 62, the climax of metamorphosis (Fig. 1B).
Figure 1.
The Expression of BRG1 But Not BAF57 Is Up-Regulated during Spontaneous Metamorphosis
RT-PCR was performed with primers specific for X. laevis BAF57 (A, C, and E) and BRG1 (B, D, and F) on total RNA isolated from whole animals, intestines, or tails of tadpoles at the indicated developmental stages, respectively (five animals for each group). The rpL8 gene served as an internal control in the same RT-PCR. Note that BRG1 mRNA levels increased at stages 62–64 in the tail as the tail resorbs (1.8-fold higher at stage 62 compared with stage 54 based on quantification using NIH ImageJ software). Higher levels of BRG1 mRNA were also observed in the intestine (1.7-fold higher at stage 62 compared with stage 54) or whole animals (1.4-fold higher at stage 62 compared with stage 54) at the climax (stage 62). BAF57 mRNA levels did not change significantly during development.
Because the expression profiles observed in the whole animal reflects the sum of expression from all the different tissues and organs in the animal, and because the expression profiles of genes may vary in each tissue/organ during metamorphosis, we were interested in determining whether the expression of BRG1 and BAF57 vary in individual organs and tissues. It is important to note that different tadpole organs and tissues undergo unique transformations, and these are developmental stage dependent. For this purpose, the expression profiles of BRG1 and BAF57 were examined in the intestine and tail. These two organs have been studied extensively at the molecular and cellular level during metamorphosis for TR-mediated gene activation (3,58,61,63). The intestine represents an organ that persists through metamorphosis but undergoes extensive remodeling, involving selective death of larval epithelial cells and proliferation and differentiation of adult cell types. In contrast, the tail represents an organ that undergoes complete resorption through apoptosis. We isolated total RNA from the intestine and tail of tadpoles at different stages of metamorphosis (five for each stage) and subjected them to RT-PCR analysis. In the intestine, BAF57 mRNA was constitutively expressed, whereas BRG1 mRNA was up-regulated during intestinal remodeling (stage 62) but returned to lower levels at the end of metamorphosis (stage 66) (Fig. 1, C and D). Similarly, in the tail, BRG1 was found to be dramatically up-regulated at stages 62 and 64, when rapid tail resorption occurs (Fig. 1F). In contrast, BAF57 mRNA levels remained unchanged during tail resorption (Fig. 1E) (stage 66 animals were not used in the expression analysis here because the tail is completely resorbed by this stage). These results suggest that the enhanced levels of BRG1 coincide with the dramatic events of intestinal remodeling and tail resorption during metamorphosis and that the expression of BRG1 is regulated by T3.
BRG1 But Not BAF57 Is a T3 Response Gene
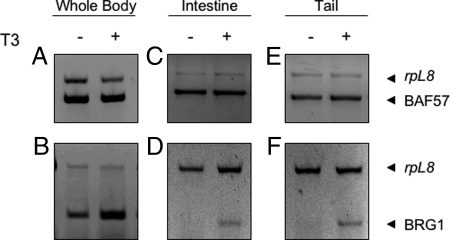
To ascertain whether T3 regulates the expression of BRG1 and BAF57, metamorphosis was precociously induced in premetamorphic tadpoles at stage 54 by treatment with 5 nm exogenous T3, a dose similar to the peak concentration during development, for three consecutive days. Such a treatment is known to induce some of the metamorphic events, such as intestinal cell death and limb morphogenesis, but not others, such as tail resorption (3,58). However, at the molecular level, many T3 response genes are induced in various organs including the tail (3,64,65,66,67,68). To determine whether the expression of BRG1 and BAF57 was affected by T3 treatment, total RNA was isolated from whole animals, intestine, and tail (five animals for each group) and subjected to RT-PCR analysis. The results showed that BRG1 expression was up-regulated in all T3-treated samples compared with untreated samples (Fig. 2, B, D, and F). In contrast, BAF57 mRNA levels were constitutively expressed with little change after T3 treatment (Fig. 2, A, C, and E). These observations are consistent with the findings during natural metamorphosis and indicate that BRG1 is regulated by T3 either directly or indirectly.
Figure 2.
T3 Treatment of Premetamorphic Tadpoles Induces BRG1 Expression but Has Little Effect on BAF57 Expression
RT-PCR was carried out with primers specific for X. laevis BRG1 and BAF57 as in Fig. 1 on total RNA isolated from whole animals, intestines, or tails of premetamorphic stage 54 tadpoles treated with or without 5 nm T3 for 3 d (five for each group). The rpL8 gene was used as the internal control.
BRG1 ATPase Activity Is Required to Enhance TR-Dependent Transcription in Xenopus Oocytes
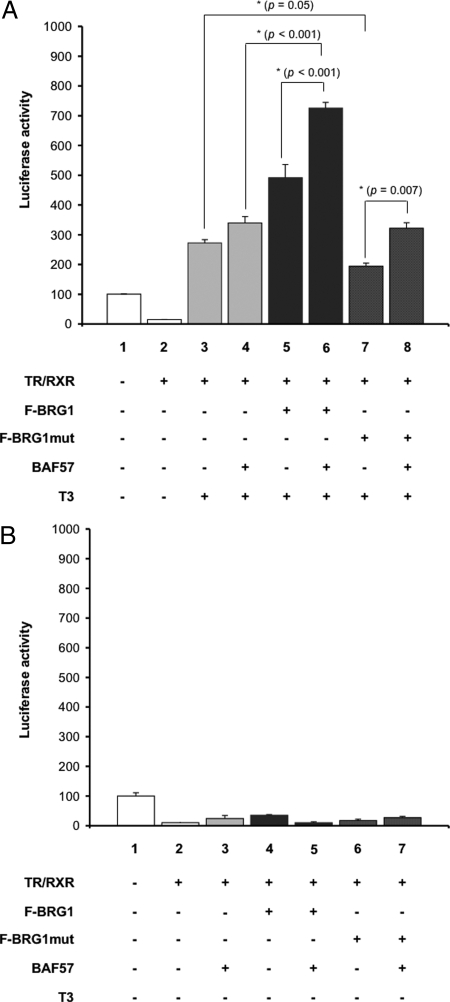
To investigate whether BRG1 can influence TR-dependent transcription, we analyzed the effect of overexpressing BRG1 on TR-mediated gene expression in the reconstituted frog oocyte system, which provides a model for studying gene regulation in the context of chromatin (69). As a reporter for T3-dependent transcriptional activity, a plasmid containing the T3-dependent promoter of Xenopus TRβA gene driving the expression of firefly luciferase (TRE-Luc) was microinjected into the oocyte nuclei together with an internal control plasmid driving the expression of Renilla luciferase. In vitro transcribed mRNAs encoding TRβ and RXRα, together with mRNAs encoding Flag-tagged BRG1 (F-BRG1) were coinjected into the cytoplasm. After overnight incubation in the presence or absence of T3, oocytes were lysed and assayed for luciferase activities. The ratio of firefly to Renilla luciferase activity was determined as a measure for transcription level from the reporter gene. In the absence of T3, overexpression of TR/RXR reduced the reporter gene transcription, and the expression of F-BRG1 did not produce any significant, reproducible effect on the reporter gene expression in the presence or absence of TR/RXR expression. This suggests that repression by unliganded TR is not affected by BRG1 overexpression (Fig. 3, columns 3 and 4) (Note that although BRG1 overexpression appeared to reduce the transcription further in Fig. 3, it enhanced or had no effect in some other experiments; e.g. see Fig. 7B below. Because the absolute luciferase activity was very low and thus relatively more variable in the presence of unliganded TR/RXR, it was difficult to observe the changes, if any, induced by BRG1 or BAF57.) In the presence of T3, F-BRG1 overexpression enhanced the activation of liganded TR in a dose-dependent manner (Fig. 3, columns 8 and 9) but not the basal expression in the absence of TR (data not shown). Because BRG1 functions as an ATP-dependent chromatin remodeling protein, we investigated whether its ATPase activity is important for enhancing TR function. Thus, we microinjected mRNA for F-BRG1mut, which contains a point mutation in its ATPase domain (K785R) that inactivated the ATPase (21). This resulted in a dose-dependent inhibition of ligand bound TR-induced reporter gene activation (Fig. 3, columns 10 and 11). These results suggest that BRG1 participates in gene activation by ligand-bound TR and that the ATPase domain of BRG1 is important for the activation.
Figure 3.
Wild-Type BRG1 Significantly Enhances TR Transcription in a Dose-Dependent Manner, whereas a Mutant BRG1 with a Mutation in the ATPase Domain Inhibits TR Transcription
The mRNA for TR (1.15 ng/oocyte) was injected into the cytoplasm of 20 oocytes together with RXR mRNA (1.15 ng/oocyte) and either the Flag-tagged BRG1 (F-BRG1) or its mutant (F-BRG1mut) mRNA (1.15 ng/oocyte or 3.5 ng/oocyte), as indicated. Subsequently, the TRE-Luc firefly luciferase reporter vector and phRG-Tk Renilla luciferase control vector was coinjected into the nucleus of the oocytes. The oocytes were incubated overnight with (lanes 7–11) or without (lanes 1–6) 50 nm T3. The oocytes were lysed and subjected to dual-luciferase assays. The relative activity of the reporter vs. that of the control was plotted with the basal activity set to 100 (lane 1). The average from five oocytes was plotted together with the se. Note that although we were not able to detect F-BRG1 or F-BRG1mut by Western blot (not shown), their effects were mRNA dose dependent, consistent with the prediction that more proteins were synthesized when more mRNA was injected. Also, although overexpression of BRG1 or its mutant appeared to reduce the transcription further in the absence of T3 (lanes 3–6), the effect was variable and not significant because the absolute luciferase activity was very low and thus relatively more variable in the presence of unliganded TR/RXR (see text and Fig. 7B).
Figure 7.
BAF57 and BRG1 Cooperatively Enhance TR-Dependent Transcription
A, The cytoplasm of oocytes was injected with TR (1.15 ng/oocyte), RXR (1.15 ng/oocyte), and BAF57 (2.3 ng/oocyte) mRNAs and/or F-BRG1 (2.3 ng/oocyte) or F-BRG1mut (2.3 ng/oocyte), as indicated. Subsequently, the TRE-Luc reporter vector together with the control Renilla luciferase vector were coinjected into the nuclei of the oocytes. The oocytes were incubated overnight in the absence (lanes 1 and 2) or presence (lanes 3–8) of 50 nm T3. The oocytes were lysed and subjected to dual-luciferase assays. The relative activity of the reporter vs. that of the control was plotted with the basal activity set to 100 (lane 1). The bars represent the means ± se of at least three independent experiments performed in quadruplicate (*, P < 0.05). B, The oocytes were injected as described in A, as indicated. After injection, the oocytes were incubated overnight in the absence of T3. The luciferase activity was plotted with the basal activity set to 100 (lane 1). Note that there seemed to be slight increases in transcription in some lanes with overexpressed BAF57 and/or BRG1. As pointed out in Fig. 3 and in the text, such effects were not significant or reproducible, likely due to the very low levels of absolute luciferase activity in the presence of unliganded TR/RXR.
BAF57 Associates with BRG1 in Xenopus Oocytes
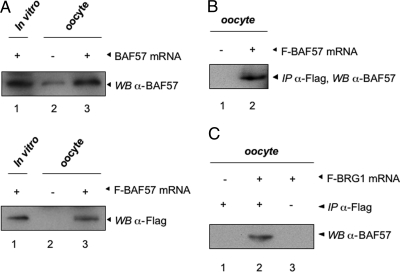
Given the involvement of BRG1 for TR function and the known association of BRG1 and BAF57 in mammalian chromatin remodeling complexes, we asked whether BRG1 also interacts with Xenopus BAF57. We first generated a polyclonal antibody against two peptides of Xenopus BAF57. To verify the specificity of the BAF57 antibody, Xenopus BAF57 with (F-BAF57) and without a Flag tag were synthesized through in vitro translation (Fig. 4A, lane 1) or after microinjecting their mRNAs into oocytes (Fig. 4A, lanes 2 and 3) and subjected to Western blot analysis. The anti-BAF57 antibody detected the overexpressed BAF57 (Fig. 4A, top panel) as well as a lower level of endogenous BAF57 (Fig. 4A, top panel, lane 2). On the other hand, the anti-Flag antibody detected signal only in lanes with overexpressed F-BAF57 but not in uninjected oocytes (Fig. 4A, bottom panel). Furthermore, immunoprecipitation (IP) with anti-Flag antibody of oocytes injected with F-BAF57 mRNA followed by Western blotting with anti-BAF57 antibody detected the overexpressed F-BAF57 in the injected but not control oocytes (Fig. 4B). These results show that the anti-BAF57 antibody recognizes endogenous BAF57 and that BAF57 is present in the frog oocyte.
Figure 4.
Endogenous BAF57 Interacts with BRG1 in the Frog Oocyte
A, A polyclonal antibody recognizes X. laevis BAF57. In vitro transcribed mRNAs for BAF57 (with or without Flag moiety) were injected into 20 oocytes. After overnight incubation, the protein extracts were isolated from the oocytes and subjected to Western blot analysis with the anti-BAF57 antibody (upper panel: lane 1, in vitro translated BAF57; lane 2, uninjected oocytes; and lane 3, oocytes injected with BAF57 mRNA with no Flag-tag) and the anti-Flag antibody [lower panel: lane 1, in vitro translated Flag-tagged BAF57 (F-BAF57); lane 2, uninjected oocytes; and lane 3, oocytes injected with F-BAF57 mRNA]. Note the presence of low levels of endogenous BAF57 (upper panel). B, To further confirm the specificity of the X. laevis BAF57 antibody, in vitro transcribed mRNA for F-BAF57 (5.75 ng/oocyte) was injected into 20 oocytes. The lysate was subjected to IP with anti-Flag affinity beads. Eluted proteins were subjected to immunoblotting with the anti-BAF57 antibody, which recognized F-BAF57 (lane 2); lane 1, uninjected oocyte lysate. C, In vitro transcribed mRNAs for Flag-tagged BRG1 (F-BRG1) (5.75 ng/oocyte) were microinjected into 20 oocytes. The oocyte extract was subjected to IP with anti-Flag affinity beads (lanes 1 and 2) or beads only (lane 3) followed by Western analysis using BAF57 antibody.
To investigate whether BRG1 interacts with BAF57 in vivo, frog oocytes were microinjected with in vitro transcribed mRNA encoding F-BRG1. After overnight incubation, the oocytes were lysed and subjected to IP with anti-Flag affinity agarose beads. Western blot analysis of the IP products showed that endogenous BAF57 was specifically coimmunoprecipitated with F-BRG1 (Fig. 4C), suggesting that BRG1 interacts with BAF57 in oocytes and that BAF57 may also participate in transcriptional regulation by TR. Further analysis is required to determine whether the interaction between BAF57 and BRG1 is functionally important.
BAF57 Enhances TR-Dependent Activity through Recruitment to the Target Promoter in Xenopus Oocytes
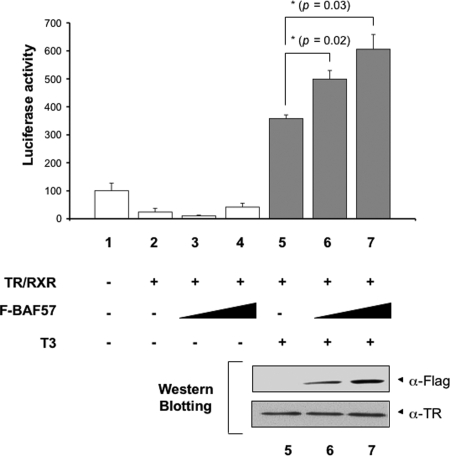
To study the involvement of BAF57 in gene activation by T3, we microinjected in vitro transcribed mRNAs encoding TR and RXR, together with mRNAs encoding F-BAF57 into the cytoplasm of frog oocytes. Luciferase assays were performed as described above. Again, unliganded TR/RXR repressed the reporter activity, and T3 led to a strong activation by TR (Fig. 5, lanes 1, 2, and 5). Overexpression of F-BAF57 enhanced transcription by liganded TR/RXR (Fig. 5, lanes 6 and 7) but had little effect in the absence of T3 (lanes 3 and 4).
Figure 5.
Overexpression of BAF57 Enhances Transcriptional Activation by TR in a Dose-Dependent Manner
The mRNAs for TR/RXR (1.15 ng/oocyte) were injected into the cytoplasm of 20 oocytes together with F-BAF57 mRNA (1.15 or 2.3 ng/oocyte) as indicated. After 4 h of incubation, the TRE-Luc reporter vector together with the control Renilla luciferase plasmid were coinjected into the nucleus of the oocytes (lanes 1–7). The oocytes were incubated overnight with or without 50 nm T3 as indicated. After incubation, the oocytes were lysed and subjected to dual-luciferase assays. The relative activity of the reporter vs. that of the control was plotted with the basal activity set to 100 (lane 1). The bars represent the means ± se of at least three independent experiments performed in triplicate (*, P < 0.05). In the lower panel, the same oocyte samples used in the luciferase assay (lanes 5–7) were subjected to Western blot analysis with the anti-Flag or TR antibody, confirming the expression of F-BAF57 and TR.
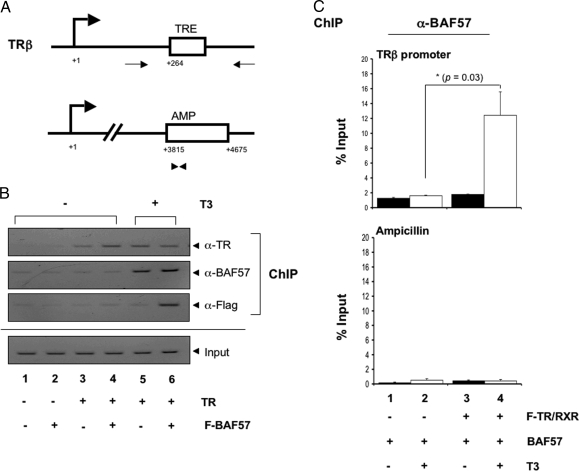
To investigate how BAF57 enhanced transcription, we analyzed the in vivo recruitment of BAF57 to the promoter with chromatin IP (ChIP) assay. Oocytes were injected with reporter DNA and mRNAs of interest and incubated overnight in the presence or absence of T3. ChIP assays were performed using antibodies against TR and BAF57. The immunoprecipitated DNA was analyzed by using either regular PCR or quantitative PCR (qPCR). For qPCR analysis, two sets of primers were used; one recognized the TRE region of the target promoter, and the other recognized a region in the ampicillin resistance gene (β-lactamase) located in the same plasmid but away from the target promoter (Fig. 6A). The latter primer served as a negative control for ChIP assay. As shown by the regular PCR in Fig. 6B, TR bound to the TRE constitutively (lanes 3–6). In the presence (Fig. 6B, lane 6) but not the absence of T3 (lanes 2 and 4), overexpressed F-BAF57 was recruited to the target promoter as shown by the ChIP assay with anti-Flag antibody. In addition, the anti-BAF57 ChIP assay showed that endogenous BAF was also recruited by ligand-bound TR (Fig. 6B, lanes 1, 2, and 5). The results were confirmed by qPCR analysis, showing that in the presence of TR, the addition of T3 increased the BAF57 recruitment to the TRE region by 10-fold (Fig. 6C) with only constitutive background signal at the control ampicillin resistance gene. Together, these data demonstrated that ligand-bound TR recruits BAF57 to the TRE for T3-induced gene activation in vivo.
Figure 6.
BAF57 Is Recruited to the TRE in the Target Promoter in the Presence of T3
A, Schematic representation of X. laevis TRβ promoter in the TRE-Luc reporter plasmid (upper panel), which is regulated by TR directly. This promoter has a TRE, as indicated. The relative location of the PCR primers used for the ChIP assays are shown as thin arrows. The transcription start site is indicated as +1. The lower panel represents the ampicillin resistance gene coding region within the same plasmid where the control qPCR primers used for the ChIP assays are represented as arrowheads. B, Ligand-bound TR/RXR recruits BAF57 to the TRE. The oocytes were injected as in Fig. 5 (F-BAF57 mRNA, 2.3 ng/oocyte). The oocytes were incubated overnight with or without 50 nm T3. The oocytes were then processed for ChIP assay with anti-Flag, anti-BAF57, and anti-TR antibodies for IP. The presence of the TRE region in the immunoprecipitated DNA was determined by PCR with target-specific primers, as indicated in A. The PCR was analyzed on a 2% agarose gel containing ethidium bromide. The DNA before IP was amplified as the input control to show that equal amounts of DNA were present in samples. Note that both endogenous (lane 5, BAF57 antibody) and overexpressed F-BAF57 (lane 6, with Flag antibody) were recruited by ligand-bound TR. C, Quantitative PCR analysis of BAF57 recruitment. The oocytes were injected as in Fig. 5, but the BAF57 mRNA contained no Flag-tag (BAF57 mRNA, 2.3 ng/oocyte). The oocytes were incubated overnight with or without 50 nm T3. The oocytes were then processed for ChIP assay with anti-BAF57 antibody for IP. Quantitative PCR was also used to analyze the recruitment of BAF57 to TRE in the presence of T3. Note the recruitment of BAF57 to the promoter in the presence of TR and T3 but not to the ampicillin gene. The average values of three independent experiments are shown ± se (*, P < 0.05).
BAF57 and BRG1 Cooperatively Enhance T3-Induced Gene Activation
Having shown that BRG1 and BAF57 could each enhance gene activation by TR, we next asked whether they could function together. We injected into frog oocytes mRNAs for TR and RXR and F-BRG1 or F-BRG1mut with or without BAF57 mRNA followed by the reporter DNA. After overnight incubation with or without T3, the oocytes were harvested for luciferase assay. Again the data showed that F-BRG1 and BAF57 enhanced the activation by ligand-bound TR individually (Fig. 7A, compare lane 3 with lanes 4 and 5), whereas F-BRG1mut inhibited TR function (Fig. 7A, compare lanes 3 and 7). When F-BRG1 and BAF57 were coexpressed, there was a synergistic enhancement of reporter activity in the presence of T3 (Fig. 7A, compare lane 6 with lanes 5 or 4). Interestingly, overexpression of BAF57 eliminated the inhibition by F-BRG1mut (Fig. 7A, compare lane 8 with 3 vs. 7 with 3). This was probably because only a fraction of the injected reporter plasmids had F-BRG1mut recruited and was thus inhibited. Endogenous wild-type BRG1 would be present at the remaining reporter plasmids and would be enhanced by the overexpressed BAF57. Thus, the inhibition of the overall luciferase activity by F-BRG1mut was offset by BAF57 overexpression. In the absence of T3, neither BRG1 nor BAF57 individually or together had any reproducible, significant effect on repression by TR in the absence of T3 (Fig. 7B), consistent with the lack of recruitment of BAF57 by TR in the absence of T3 (Fig. 6). Taken together, these results suggest that BAF57 contributes to gene regulation by ligand-bound TR cooperatively with BRG1, likely via enhanced formation of the chromatin remodeling complex.
DISCUSSION
Thyroid hormone (T3) is critical for vertebrate development. T3 exerts most of its physiological functions through TRs in the nucleus. A wealth of studies in vitro and in cell cultures has demonstrated that both ATP-independent histone modifications and ATP-dependent chromatin remodeling play important roles in gene regulation by nuclear hormone receptors, including T3-induced gene activation. However, relatively few studies have addressed the function of such chromatin modification/remodeling complexes in TR function in vivo. As a first step toward investigating the role of ATP-dependent chromatin remodeling complexes in TR-controlled gene expression in vivo during vertebrate development, we have shown here that X. laevis BRG1, an ATP-dependent chromatin remodeling factor, is up-regulated by T3 in not only the whole animal but also in specific organs such as the tail and intestine during metamorphosis whereas one of its binding proteins in the chromatin remodeling complexes, BAF57, is constitutively expressed. We have further shown in the reconstituted frog oocyte transcription system, where we can study TR function in the context of chromatin, that BRG1 and BAF57 synergistically enhance gene activation by T3-bound TR. Hence, our data support a role for BAF57 and BRG1-containing ATP-dependent chromatin remodeling complexes in mediating transcriptional activation by ligand-bound TR during vertebrate development.
BRG1 and BAF57 Contributes Synergistically to TR-Regulated Gene Expression in the Presence of T3 in Vivo
BRG1 and its related protein BRM are large helicase-like ATPases in the class of chromatin complexes of about 2 MDa that are the functional homologs of the SWI/SNF complex in Saccharomyces cerevisiae (70,71). There is growing evidence indicating that they play crucial roles in the regulation of cell growth and differentiation, and in animal development and tumorigenesis, with most of these studies focused in yeast and Drosophila (24,25,30,72,73). These chromatin-remodeling complexes participate in both activation and repression of various genes. Our transcriptional analysis in Xenopus oocytes showed that overexpression of BRG1 or its mutant that lacks ATPase activity (BRG1mut) did not affect the promoter activity in the absence of T3. However, overexpression of BRG1 enhanced gene activation by T3-bound TR, whereas the mutant BRG1 functioned as a dominant negative by inhibiting TR activation in the presence of T3, suggesting that ATP-dependent chromatin remodeling is necessary for gene activation by ligand-bound TR. Our findings here are consistent with earlier studies using a fusion protein of Gal4 DNA-binding domain and the ligand-binding domain of TR (21).
More importantly, we showed that BAF57, a 57-kDa BRG1 complex subunit, also enhanced the function of ligand-bound TR through direct recruitment to the target promoter by TR. Furthermore, BAF57 interacted with BRG1 in vivo and synergistically activated the reporter gene with BRG1 in the presence of ligand-bound TR. In addition, BRG1 is also recruited to the target promoter by Gal4-TR fusion protein in the presence of T3 (21). Thus, our studies suggest for the first time that BAF57 is recruited by TR to activate its target gene in the presence of its ligand, and the synergy between BAF57 and BRG1 supports the model that both function together in the SWI/SNF complex to facilitate chromatin remodeling during gene activation by ligand-bound TR.
A Role of BRG1-BAF57-Containing Chromatin Remodeling Complex in Gene Regulation by TR and Postembryonic Vertebrate Development
As a broadly expressed core subunit of chromatin remodeling complexes, BRG1 may be expected to participate in diverse developmental processes. Indeed, previous studies with Xenopus oocytes have shown that BRG1 is needed for processes requiring nucleosome remodeling during oogenesis (74). Xenopus BRG1 also plays an important role in the developmental processes that are activated upon fertilization because many of these, such as decondensation of the densely packed sperm and the maternal chromosomes as well as gene activation, require remodeling of the chromatin structure (75,76). In addition, BRG1 has been reported to be essential for the proliferation of mouse embryonic F9 cells (77). Furthermore, BRG1 gene deletion in mice is lethal at the periimplantation stage, whereas BRG1 heterozygote mice are predisposed to exencephaly and tumors of epithelial origin, showing the importance of BRG1 in early development and diseases (75,78,79). The expression profile of BAF57 appears to be similar to that reported for BRG1 during vertebrate development (80). Currently, there have been few mechanistic studies on the roles of BRG1 and BAF57 during vertebrate development due to lethality of the BRG1 knockout mice in mammals (79).
Amphibian metamorphosis provides a unique opportunity to investigate gene function during development largely because of the ability to manipulate this process in live animals independent of maternal regulations. Our expression studies have shown that both BAF57 and BRG1 are expressed during amphibian metamorphosis. Although BAF57 is expressed constitutively, BRG1 expression is highly up-regulated during both natural and T3-induced metamorphosis, consistent with the finding that BRG1 was among the T3 regulated genes from a cDNA array analysis of the tadpole intestine (65). More importantly, BRG1 up-regulation correlates temporally with the metamorphic transitions in different organs. These findings suggest that both BRG1 and BAF57 participate in gene regulation during metamorphosis.
The total dependence of amphibian metamorphosis on T3 suggests that the function of BRG1 and BAF57 during metamorphosis is likely through their regulation of the transcriptional activity of TR during development. Our studies in oocytes as discussed above indicate that BRG1 and BAF57 are capable of enhancing gene activation by ligand-bound TR directly through recruitment of these factors to TR target promoters. The activated TR will lead to higher levels of BRG1, which will then further enhance TR activity. A direct in vivo test of these would require analysis of their recruitment by TR to endogenous target genes during metamorphosis. This would require the development of antibodies that will allow us to detect endogenous BRG1 during frog development and, more importantly, to analyze the association of BRG1 and BAF57 with endogenous target genes by using ChIP assays on tadpole tissues. In addition, future studies using genetic approaches, such as overexpressing the mutant BRG1 to study the effect on TR-dependent gene regulation and metamorphosis may allow us to directly assess the contributions of these ATP-dependent chromatin remodeling factors during vertebrate development.
MATERIALS AND METHODS
Experimental Animals
All experiments involving X. laevis animals described herein were conducted in accord with accepted standards of humane animal care and approved by the National Institute of Child Health and Human Development Animal Use and Care Committee. Adult and tadpoles of X. laevis were reared in the laboratory or purchased from NASCO (Fort Atkinson, WI). Tadpole developmental stages were determined according to Nieuwkoop and Faber (81) and were killed at indicated stages for RNA isolation. Adult female frogs were used for oocyte preparation.
RNA Isolation and Analysis
Total RNA was isolated from whole tadpoles or dissected tadpole intestine and tail at different developmental stages and during T3-induced metamorphosis (five for each group). For T3-induced metamorphosis, stage 54 premetamorphic tadpoles were treated in their rearing water with 5 nm T3 for 3 d at 18 C. RNA was isolated from whole tadpoles or the dissected tissue samples using Trizol (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. For gene expression analysis, RT-PCRs were carried out using the Superscript One-Step RT-PCR kit (Invitrogen). The ribosomal protein L8 (rpl8) was used as an internal control (82). The sequences of the primers used were 5′-CGTGGTGCTCCTCTTGCCAAG-3′ (forward) and 5′-GACGACCAGTACGACGAGCAG-3′ (reverse) for rpl8, 5′-TGCTTATCTCGCTTACATCAGTGC-3′ (forward) and 5′-CACAGCGCCGCTTCAACTCATTG-3′ (reverse) for BAF57 (80), and 5′-GAACACTTGGGATTCACAGGA-3′ (forward) and 5′-TCATCTGCTTTGGTGGTGCC-3′ (reverse) for BRG1 (75). All primers were designed to include more than one exon to avoid signals from potential genomic DNA contamination. RT-PCRs were carried out with SuperScript II (Invitrogen) for 30 min at 50 C for RT, followed by 24–28 cycles of 94 C for 30 sec, 55 C for 30 sec, and 72 C for 30 sec. The primer concentrations were 0.04 μm for rpl8 and 0.2–0.4 μm for the other genes, and 0.5 μg RNA was added to each reaction mixture. The amplified PCR products were analyzed on an agarose gel (1.5% agarose-Tris-borate-EDTA gel with a 0.5× Tris-borate-EDTA buffer system) by electrophoresis and stained with ethidium bromide. All reactions were repeated at least three times with similar results.
Plasmid Constructs
The full-length Xenopus BAF57 was obtained by PCR with the following primers: forward, 5′-CATATGTCCAAGCGACCATCG-3′, and reverse, 5′-GCGGCCGCGTTCTTGTGATCAGGCTCTTC-3′. The BAF57 construct was generated to encode a fusion protein with an N-terminal Flag epitope tag, followed by the simian virus 40 nuclear localization signal using a 5′ primer containing the sequences encoding these fusion peptides in frame with the Xenopus BAF57 coding sequence. To facilitate the cloning, the restriction site for BglII or SpeI was added to the 5′ end of the forward primer or reverse primer, respectively. The primers used were forward primer 5′-AGATCTATGGACTACAAAGACGATGACGATAAAGGATCCCCAAAGAAGAAGCGTAAGGTACTCGAG ATGTCCAAGCGACCATCGTATGC-3′ (the Flag-tag is underlined, and the nuclear localization signal is shown in boldface) and reverse primer 5′-ACTAGTGTTCTTGTGATCAGGCTCTTC-3′. For expression and detection in frog oocytes, the PCR product was cloned into the BglII and SpeI sites of the T7Ts expression vector, which contains the T7 RNA polymerase promoter driving the expression of the cloned gene (86).
The expression plasmids for F-BRG1 and a dominant-negative version of BRG1 (F-BRG1mut) under the control of the SP6 promoter were kindly donated by Dr. J. Wong (East China Normal University) (21). The F-BRG1mut expression plasmid contains a mutation in the highly conserved ATP-binding pocket (K785R) (21). This mutation is sufficient to inactivate ATPase activity of BRG1. Because there is greater than 85% homology shared between human and Xenopus BRG1, of which 92% homology is shared in the ATP-binding motif that was targeted by mutagenesis in F-BRG1mut (data not shown), and because many proteins are known to function interchangeably with homologs sharing even 70% or less among different species, the human proteins were expected to work properly in frog oocytes.
Antibodies
Mouse anti-Flag M2 monoclonal antibody and Ezview Red Anti-Flag M2 affinity gel were purchased from Sigma Chemical Co. (St. Louis, MO). Rabbit anti-Xenopus TR polyclonal antibody (PB) has previously been described (69). The anti-Xenopus BAF57 polyclonal antibodies were generated by coinjecting two peptides derived from X. laevis BAF57, ASSGITIPKPPKPPDKPLMP (amino acids 53–72; keyhole limpet hemocyanin-conjugated) and DRHQDKKRKFLESTESFNNE (amino acids 250–269; keyhole limpet hemocyanin-conjugated) into rabbits (Invitrogen).
Transcription Assay in the Xenopus Oocyte System
The pSP64 plasmids containing TR and RXR cDNAs, which encode Xenopus TRβA and RXRα, were linearized and transcribed in vitro using an SP6 kit (Ambion, Austin, TX) and have previously been described (69). The pGL-TRE luciferase reporter vector (TRE-Luc) contains the T3-dependent promoter of the X. laevis TRβA gene driving the expression of the firefly luciferase (83).
The T7Ts-Flag-BAF57, T7Ts-Flag-BRG1, and T7Ts-Flag-BRG1mut were used to synthesize the corresponding mRNAs in vitro with a T7 in vitro transcription kit (mMESSAGE mMACHINE; Ambion). Microinjection experiments were performed as described previously (69). Briefly, the cytoplasm of stage VI oocytes from X. laevis was injected with the indicated mRNAs (1.15 ng/oocyte for TR and RXR and 1.15–5.75 ng/oocyte for F-BAF57, F-BRG1, and F-BRG1mut). The luciferase reporter TRE-Luc plasmid DNA (0.33 ng/oocyte) and the control vector, phRG-TK (0.03 ng/oocyte), which contained the Herpes simplex virus tk promoter driving the expression of the Renilla luciferase, were coinjected into the nucleus 6 h after mRNA injection to ensure protein expression before chromatin assembly. The injected oocytes were incubated at 18 C overnight in modified Barth's solution-high salt buffer (69) in the presence or absence of 50 nm T3 (Fig. 3). After incubation, the injected oocytes were prepared for luciferase assay using the Dual-Luciferase-Reporter Assay system (Promega, Madison, WI) according to the manufacturer's protocol. Six oocytes were lysed by pipetting in 90 μl 1× lysis buffer (Promega), and 7 μl of the lysate was used for each luciferase assay. Triplicate assays were performed at the same time. The relative expression of firefly luciferase from the reporter plasmid to Renilla luciferase from the control plasmid was determined and is reported here. Each data point represents the average from a group that was assayed in triplicate. These experiments were repeated five times, and intergroup comparisons were performed with the one-way ANOVA followed by Fisher's protected least significant difference test. Data are represented as the mean ± se; a P value ≤ 0.05 was considered to be statistically significant. A portion of the oocyte lysate was subjected to Western blot analysis to verify protein expression from the injected mRNAs.
IP
The indicated mRNA (5.75 ng/oocyte) was microinjected into the cytoplasm of 20 X. laevis stage VI oocytes and incubated overnight in the presence or absence of 50 nm T3 at 18 C. After incubation, the oocytes were lysed by pipetting in 400 μl IP buffer [20 mm HEPES (pH 7.5), 5 mm KCl, 1.5 mm MgCl2, 1 mm EGTA, 10 mm β-glycerophosphate, 150 mm NaCl, 0.1% Nonidet P-40, 1 mm dithiothreitol, 0.2 mm phenylmethylsulfonyl fluoride, protease inhibitor cocktail; Roche, Indianapolis, IN]. The lysate was centrifuged twice at 12,000 rpm at 4 C for 15 min to remove yolk proteins and debris. The lysate was precleared with sperm DNA agarose beads (Upstate, Lake Placid, NY) and 20 μl protein A- and 20 μl protein G-Sepharose (Amersham, Piscataway, NJ) in 0.5 ml IP buffer for 1 h at 4 C. The lysate was used for IP with Ezview Red anti-Flag M2 affinity gel (Sigma) or antibody-cross-linked protein agarose beads (10 μl). Each lysate was incubated with the gel or indicated antibody-cross-linked protein agarose beads overnight at 4 C. After incubation, the gel or beads were washed three times with IP buffer, resuspended in 40 μl 2× SDS-PAGE gel loading buffer, and boiled at 95 C for 5 min to denature the proteins. The bound proteins were separated on an SDS-polyacrylamide gel and immunoblotted with indicated antibodies.
ChIP Assays
ChIP assays using X. laevis oocytes were performed as previously described (84,85). Twenty X. laevis oocytes were used for each assay. The following antibodies were used in the assay: anti-Xenopus TR, anti-Xenopus BAF57, and Ezview Red anti-Flag M2 affinity gel (Sigma). After reversal of the DNA-protein cross-links, purification of the immunoprecipitated DNA was carried out with a PCR purification kit (QIAGEN, Valencia, CA). Buffer EB (40 μl) was used for elution, and 4 μl of the DNA from the ChIP assay was analyzed by routine PCR or qPCR. PCR products from routine PCR were resolved by electrophoresis on 1% agarose gels and viewed with ethidium bromide staining. Quantitative PCR was performed in duplicate on an ABI 7000 (Applied Biosystems, Foster City, CA) system using the promoter-specific primers and 6-carboxyfluorescein-labeled TaqMan probes (Applied Biosystems). For each qPCR assay, 10 3-fold serial dilutions of the TRE region of the T3-dependent TRβA promoter input DNA served as the standards for the quantification of the experimental samples. The calculated standard curves ranged in slope from −3.30 to −3.50, where the theoretical amplification has a slope of −3.32. The data from the experimental samples were within the range of the standard curve. Also included in the analysis were the chromatin input samples (before IP). These samples served as an input control for the qPCR amplification. The primers used for the quantitative PCR were as follows: 5′-CCCCTATCCTTGTTCGTCCTC-3′ (forward) and 5′-GCGCTGGGCTGTCCT-3′ (reverse) for the TRE region of the TRβA promoter and 5′-GCCGAGCGCAGAAGTG-3′ (forward) and 5′-TCTAGCTTCCCGGCAACAATTAA-3′ (reverse) for the ampicillin resistance gene. The 6-carboxyfluorescein-labeled probes were 5′-CCTAGGCAGGTCATTTC-3′ and 5′-ATGAGGCTGAGCATTCA-3′ for the TRβA promoter and ampicillin resistance gene, respectively. All ChIP experiments were repeated three to four times with similar results. Intergroup comparisons were performed with ANOVA followed by Fisher's protected least significant difference test; a P value ≤ 0.05 was considered to be statistically significant.
Acknowledgments
We thank Dr. J. Wong for the BRG1 and BRG1mut expression constructs.
Footnotes
This research was supported in part by the Intramural Research Program of the National Institute of Child Health and Human Development, National Institutes of Health (NIH), and in part by the NIH and the National Center for Research Resources Grant P20 RR16456.
Disclosure Statement: The authors have nothing to disclose.
First Published Online January 31, 2008
Abbreviations: BAF, BRG1-associated factor; BRG1, Brahma-related gene 1; BRM, Brahma; ChIP, chromatin immunoprecipitation; F-BRG1, Flag-tagged BRG1; IP, immunoprecipitation; qPCR, quantitative PCR; RXR, 9-cis-retinoic acid receptor; SWI/SNF, mating type switching/sucrose nonfermenting; TR, thyroid hormone receptor; TRE, T3 response element.
References
- Cheng SY 2003 Thyroid hormone receptor mutations in cancer. Mol Cell Endocrinol 213:23–30 [DOI] [PubMed] [Google Scholar]
- Lazar MA 1993 Thyroid hormone receptors: multiple forms, multiple possibilities. Endocr Rev 14:184–193 [DOI] [PubMed] [Google Scholar]
- Shi YB 1999 Amphibian metamorphosis: from morphology to molecular biology. New York: John Wiley, Sons [Google Scholar]
- Yen PM 2001 Physiological and molecular basis of thyroid hormone action. Physiol Rev 81:1097–1142 [DOI] [PubMed] [Google Scholar]
- Flamant F, Samarut J 2003 Thyroid hormone receptors: lessons from knockout and knock-in mutant mice. Trends Endocrinol Metab 14:85–90 [DOI] [PubMed] [Google Scholar]
- Oppenheimer JH, Schwartz HL, Mariash CN, Kinlaw WB, Wong NC, Freake HC 1987 Advances in our understanding of thyroid hormone action at the cellular level. Endocr Rev 8:288–308 [DOI] [PubMed] [Google Scholar]
- Samuels HH, Forman BM, Horowitz ZD, Ye S 1988 Regulation of gene expression by thyroid hormone. J Clin Invest 81:957–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RM 1988 The steroid and thyroid hormone receptor superfamily. Science 240:889–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P 1995 The nuclear receptor superfamily: the second decade. Cell 83:835–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai MJ, O'Malley BW 1994 Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem 63:451–486 [DOI] [PubMed] [Google Scholar]
- Wong J, Shi YB, Wolffe AP 1995 A role for nucleosome assembly in both silencing and activation of the Xenopus TRβA gene by the thyroid hormone receptor. Genes Dev 9:2696–2711 [DOI] [PubMed] [Google Scholar]
- Wolffe AP 1997 Chromatin remodeling regulated by steroid and nuclear receptors. Cell Res 7:127–142 [DOI] [PubMed] [Google Scholar]
- Wolffe AP, Wong J, Li Q, Levi BZ, Shi YB 1997 Three steps in the regulation of transcription by the thyroid hormone receptor: establishment of a repressive chromatin structure, disruption of chromatin and transcriptional activation. Biochem Soc Trans 25:612–615 [DOI] [PubMed] [Google Scholar]
- Fondell JD, Roy AL, Roeder RG 1993 Unliganded thyroid hormone receptor inhibits formation of a functional preinitiation complex: implications for active repression. Genes Dev 7:1400–1410 [DOI] [PubMed] [Google Scholar]
- Ito M, Roeder RG 2001 The TRAP/SMCC/mediator complex and thyroid hormone receptor function. Trends Endocrinol Metab 12:127–134 [DOI] [PubMed] [Google Scholar]
- Rachez C, Freedman LP 2000 Mechanisms of gene regulation by vitamin D3 receptor: a network of coactivator interactions. Gene 246:9–21 [DOI] [PubMed] [Google Scholar]
- Zhang J, Lazar MA 2000 The mechanism of action of thyroid hormones. Annu Rev Physiol 62:439–466 [DOI] [PubMed] [Google Scholar]
- Burke LJ, Baniahmad A 2000 Co-repressors 2000. FASEB J 14:1876–1888 [DOI] [PubMed] [Google Scholar]
- Jepsen K, Rosenfeld MG 2002 Biological roles and mechanistic actions of co-repressor complexes. J Cell Sci 115:689–698 [DOI] [PubMed] [Google Scholar]
- Jones PL, Shi YB 2003 N-CoR-HDAC corepressor complexes: roles in transcriptional regulation by nuclear hormone receptors. Curr Top Microbiol Immunol 274:237–268. [DOI] [PubMed] [Google Scholar]
- Huang Z-Q, Li J, Sachs LM, Cole PA, Wong J 2003 A role for cofactor-cofactor and cofactor-histone interactions in targeting p300, SWI/SNF and Mediator for transcription. EMBO J 22:2146–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna NJ, O'Malley BW 2001 Nuclear receptors, coregulators, ligands, and selective receptor modulators: making sense of the patchwork quilt. Ann NY Acad Sci 949:3–5 [DOI] [PubMed] [Google Scholar]
- Rachez C, Freedman LP 2001 Mediator complexes and transcription. Curr Opin Cell Biol 13:274–280 [DOI] [PubMed] [Google Scholar]
- Becker PB, Horz W 2002 ATP-dependent nucleosome remodeling. Annu Rev Biochem 71:247–273 [DOI] [PubMed] [Google Scholar]
- Muchardt C, Yaniv M 2001 When the SWI/SNF complex remodels. The cell cycle. Oncogene 20:3067–3075 [DOI] [PubMed] [Google Scholar]
- Yoshinaga SK, Peterson CL, Herskowitz I, Yamamoto KR 1992 Roles of SWI1, SWI2, and SWI3 proteins for transcriptional enhancement by steroid receptors. Science 258:1598–1604 [DOI] [PubMed] [Google Scholar]
- Lemon B, Inouye C, King DS, Tjian R 2001 Selectivity of chromatin-remodeling cofactors for ligand activated transcription. Nature 414:924–928 [DOI] [PubMed] [Google Scholar]
- Muchardt C, Yaniv M 1993 A human homologue of Saccharomyces cerevisiae SNF2/SWI2 and Drosophila brm genes potentiates transcriptional activation by the glucocorticoid receptor. EMBO J 12:4279–4290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadam S, Emerson BM 2002 Mechanisms of chromatin assembly and transcription. Curr Opin Cell Biol 14:262–268 [DOI] [PubMed] [Google Scholar]
- Martens JA, Winston F 2003 Recent advances in understanding chromatin remodeling by SWI/SNF complexes. Curr Opin Genet Dev 13:136–142 [DOI] [PubMed] [Google Scholar]
- Wang W, Chi T, Xue Y, Zhou S, Kuo A, Crabtree GR 1998 Architectural DNA binding by a high-mobility-group/kinesin-like subunit in mammalian SWI/SNF-related complexes. Proc Natl Acad Sci USA 95:492–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman JL, Kingston RE 1998 Alteration of nucleosome structure as a mechanism of transcriptional regulation. Annu Rev Biochem 67:545–579 [DOI] [PubMed] [Google Scholar]
- Muchardt C, Yaniv M 1999 ATP-dependent chromatin remodelling: SWI/SNF and Co. are on the job. J Mol Biol 293:187–198 [DOI] [PubMed] [Google Scholar]
- Badenhorst P, Voas M, Rebay I, Wu C 2002 Biological functions of the ISWI chromatin remodeling complex NURF. Genes Dev 16:3186–3198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignali M, Hassan AH, Neely KE, Workman JL 2000 ATP-dependent chromatin-remodeling complexes. Mol Cell Biol 20:1899–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan AH, Neely KE, Vignali M, Reese JC, Workman JL 2001 Promoter targeting of chromatin-modifying complexes. Front Biosci 6:D1054–D1064 [DOI] [PubMed] [Google Scholar]
- Li B, Carey M, Workman JL 2007 The role of chromatin during transcription. Cell Res 128:707–719 [DOI] [PubMed] [Google Scholar]
- Dilworth FJ, Fromental-Ramain C, Yamamoto K, Chambon P 2000 ATP-driven chromatin remodeling activity and histone acetyltransferases act sequentially during transactivation by RAR/RXR in vitro. Mol Cell 6:1049–1058 [DOI] [PubMed] [Google Scholar]
- Shen X, Xiao H, Ranallo R, Wu WH, Wu C 2003 Modulation of ATP-dependent chromatin-remodeling complexes by inositol polyphosphates. Science 3299:112–114 [DOI] [PubMed] [Google Scholar]
- Wallberg AE, Neely KE, Hassan AH, Gustafsson JA, Workman JL, Wright AP 2000 Recruitment of the SWI-SNF chromatin remodeling complex as a mechanism of gene activation by the glucocorticoid receptor τ1 activation domain. Mol Cell Biol 20:2004–2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Zhang B, Faller DV 2004 BRG1/BRM and prohibitin are required for growth suppression by estrogen antagonists. EMBO J 23:2293–2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote J, Peterson CL, Workman JL 1998 Perturbation of nucleosome core structure by the SWI/SNF complex persists after its detachment, enhancing subsequent transcription factor binding. Proc Natl Acad Sci USA 95:4947–4952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer CJ, Archer TK 1998 Chromatin remodeling by the glucocorticoid receptor requires the BRG1 complex. Nature 393:88–91 [DOI] [PubMed] [Google Scholar]
- Ostlund Farrants AK, Blomquist P, Kwon H, Wrange O 1997 Glucocorticoid receptor-glucocorticoid response element binding stimulates nucleosome disruption by the SWI/SNF complex. Mol Cell Biol 17:895–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi TH, Wan M, Zhao K, Taniuchi I, Chen L, Littman DR, Crabtree GR 2002 Reciprocal regulation of CD4/CD8 expression by SWI/SNF-like BAF complexes. Nature 418:195–199 [DOI] [PubMed] [Google Scholar]
- Nie Z, Xue Y, Yang D, Zhou S, Deroo BJ, Archer TK, Wang W 2000 A specificity and targeting subunit of a human SWI/SNF family-related chromatin-remodeling complex. Mol Cell Biol 20:8879–8888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Xue Y, Zhou S, Kuo A, Cairns BR, Crabtree GR 1996 Diversity and specialization of mammalian SWI/SNF complexes. Genes Dev 10:2117–2130 [DOI] [PubMed] [Google Scholar]
- Wang W, Cote J, Xue Y, Zhou S, Khavari PA, Biggar SR, Muchardt C, Kalpana GV, Goff SP, Yaniv M, Workman JL, Crabtree GR 1996 Purification and biochemical heterogeneity of the mammalian SWI-SNF complex. EMBO J 15:5370–5382 [PMC free article] [PubMed] [Google Scholar]
- Chen J, Archer TK 2005 Regulating SWI/SNF subunit levels via protein-protein interactions and proteasomal degradation: BAF155 and BAF170 limit expression of BAF57. Mol Cell Biol 25:9016–9027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S, Yun R, Datta A, Lacomis L, Erdjument-Bromage H, Kumar J, Tempst P, Sif S 2003 mSin3A/histone deacetylase 2- and PRMT5-containing Brg1 complex is involved in transcriptional repression of the Myc target gene cad. Mol Cell Biol 23:7475–7487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao PW, Fryer CJ, Trotter KW, Wang W, Archer TK 2003 BAF60a mediates critical interactions between nuclear receptors and the BRG1 chromatin-remodeling complex for transactivation. Mol Cell Biol 23:6210–6220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belandia B, Oxford RL, Hurst HC, Parker MG 2002 Targeting of SWI/SNF chromatin remodeling complexes to estrogen-responsive genes. EMBO J 21:4094–4103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiskinis E, Garcia-Pedrero JM, Villaronga MA, Parker MG, Belandia B 2006 Identification of BAF57 mutations in human breast cancer cell lines. Breast Cancer Res Treat 98:191–198 [DOI] [PubMed] [Google Scholar]
- Garcia-Pedrero JM, Kiskinis E, Parker MG, Belandia B 2006 The SWI/SNF chromatin remodeling subunit BAF57 is a critical regulator of estrogen receptor function in breast cancer cells. J Biol Chem 281:22656–22664 [DOI] [PubMed] [Google Scholar]
- Link KA, Burd CJ, Williams E, Marshall T, Rosson G, Henry E, Weissman B, Knudsen KE 2005 BAF57 governs androgen receptor action and androgen-dependent proliferation through SWI/SNF. Mol Cell Biol 25:2200–2215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descrisofaro MF, Betz BL, Rorie CJ, Reisman DN, Wang W, Weissman BE 2001 Characterization of SWI/SNF protein expression in human breast cancer cell lines and other malignancies. J Cell Physiol 186:136–145 [DOI] [PubMed] [Google Scholar]
- Versteege I, Sevent N, Lange J, Rousseau-Merck MF, Ambros P, Handgretinger R, Aurias A, Delattre O 1998 Truncating mutations of hSNF5/INI1 in aggressive paediatric cancer. Nature 394:203–206 [DOI] [PubMed] [Google Scholar]
- Dodd MHI, Dodd JM 1976 The biology of metamorphosis. In: Lofts B, ed. Physiology of the amphibia. New York: Academic Press; 467–599 [Google Scholar]
- Schreiber AM, Das B, Huang H, Marsh-Armstrong N, Brown DD 2001 Diverse developmental programs of Xenopus laevis metamorphosis are inhibited by a dominant negative thyroid hormone receptor. Proc Natl Acad Sci USA 98:10739–10744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz DR, Tomita A, Fu L, Paul BD, Shi YB 2004 Transgenic analysis reveals that thyroid hormone receptor is sufficient to mediate the thyroid hormone signal in frog metamorphosis. Mol Cell Biol 24:9026–9037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz DR, Paul BD, Fu L, Shi YB 2006 Molecular and developmental analyses of thyroid hormone receptor function in Xenopus laevis, the African clawed frog. Gen Comp Endocrinol 145:1–19 [DOI] [PubMed] [Google Scholar]
- Buchholz DR, Hsia SC, Fu L, Shi YB 2003 A dominant negative thyroid hormone receptor blocks amphibian metamorphosis by retaining corepressors at target genes. Mol Cell Biol 23:6750–6758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizato K 1989 Biochemistry and cell biology of amphibian metamorphosis with a special emphasis on the mechanism of removal of larval organs. Int Rev Cytol 119:97–149 [DOI] [PubMed] [Google Scholar]
- Denver RJ, Pavgi S, Shi YB 1997 Thyroid hormone-dependent gene expression program for Xenopus neural development. J Biol Chem 272:8179–8188 [DOI] [PubMed] [Google Scholar]
- Buchholz DR, Heimeier RA, Das B, Washington T, Shi YB 2006 Pairing morphology with gene expression in thyroid hormone-induced intestinal remodeling and identification of a core set of TH-induced genes across tadpole tissues. Dev Biol 303:576–590 [DOI] [PubMed] [Google Scholar]
- Das B, Cai L, Carter MG, Piao YL, Sharov AA, Ko MSH, Brown DD 2006 Gene expression changes at metamorphosis induce by thyroid hormone in Xenopus laevis tadpoles. Dev Biol 291:342–355 [DOI] [PubMed] [Google Scholar]
- Amano T, Yoshizato K 1998 Isolation of genes involved in intestinal remodeling during anuran metamorphosis. Wound Repair Regen 6:302–313 [DOI] [PubMed] [Google Scholar]
- Shi Y-B 1996 Thyroid hormone-regulated early and late genes during amphibian metamorphosis. In: Gilbert LI, Tata JR, Atkinson BG, eds. Metamorphosis: post-embryonic reprogramming of gene expression in amphibian and insect cells. New York: Academic Press; 505–538 [Google Scholar]
- Wong J, Shi YB 1995 Coordinated regulation of and transcriptional activation by Xenopus thyroid hormone and retinoid X receptors. J Biol Chem 270:18479–18483 [DOI] [PubMed] [Google Scholar]
- Stern MJ, Jensen RE, Herskowitz I 1984 Five SWI genes are required for expression of the HO gene in yeast. J Mol Biol 178:853–868 [DOI] [PubMed] [Google Scholar]
- Niegeborn L, Carlson M 1984 Genes affecting the regulation of SUC2 gene expression in Saccharomyces cerevisiae. Genetics 108:845–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller C, Leutz A 2001 Chromatin remodeling in development and differentiation. Curr Opin Genet Dev 11:167–174 [DOI] [PubMed] [Google Scholar]
- Tamkun JW 1995 The role of brahma and related proteins in transcription and development. Curr Opin Genet Dev 5:473–477 [DOI] [PubMed] [Google Scholar]
- Gelius B, Wade P, Wolffe A, Wrange O, Ostulund Farrants AK 1999 Characterization of chromatin remodeling activity in Xenopus oocytes. Eur J Biochem 262:426–434 [DOI] [PubMed] [Google Scholar]
- Seo S, Richarson GA, Kroll KL 2005 The SWI/SNF chromatin remodeling protein Brg1 is required for vertebrate neurogenesis and mediates transactivation of Ngn and NeuroD. Development 132:105–115 [DOI] [PubMed] [Google Scholar]
- Seo S, Herr A, Lim JW, Richardson GA, Richardson H, Kroll KL 2005 Geminin regulates neuronal differentiation by antagonizing Brg1 activity. Genes Dev 19:1723–1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumi-Ichinose C, Ichinose H, Metzger D, Chambon P 1997 SNF2β-BRG1 is essential for the viability of F9 murine embryonal carcinoma cells. Mol Cell Biol 17:5976–5986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto S, Banine F, Struve J, Xing R, Adams C, Liu Y, Metzger D, Chambon P, Rao MS, Sherman LS 2005 BRG1 is required for murine neural stem cell maintenance and gliogenesis. Dev Biol 289:372–383 [DOI] [PubMed] [Google Scholar]
- Bultman S, Gebuhr T, Yee D, La Mantia C, Nicholson J, Gilliam A, Randazzo F, Metzger D, Chambon P, Crabtree G, Magnuson T 2000 A Brg1 null mutation in the mouse reveals functional differences among mammalian SWI/SNF complexes. Mol Cell 6:1287–1295 [DOI] [PubMed] [Google Scholar]
- Domingos PM, Obukhanych TV, Altmann CR, Hemmati-Brivanlou A 2002 Cloning and developmental expression of BAF57 in Xenopus laevis. Mech Dev 116:177–181 [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J 1965 Normal table of Xenopus laevis. Amsterdam: North Holland Publishing [Google Scholar]
- Shi YB, Liang VC 1994 Cloning and characterization of the ribosomal protein L8 gene from Xenopus laevis. Biochim Biophys Acta 1217:227–228 [DOI] [PubMed] [Google Scholar]
- Amano T, Leu K, Yoshizato K, Shi YB 2002 Thyroid hormone regulation of a transcriptional coactivator in Xenopus laevis: implication for a role in postembryonic tissue remodeling. Dev Dyn 223:526–535 [DOI] [PubMed] [Google Scholar]
- Hsia SC, Shi YB 2002 Chromatin disruption and histone acetylation in the regulation of HIV-LTR by thyroid hormone receptor. Mol Cell Biol 22:4043–4052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita A, Buchholz DR, Shi YB 2004 Recruitment of N-CoR/SMRT-TBLR1 corepressor complex by unliganded thyroid hormone receptor for gene repression during frog development. Mol Cell Biol 24:3337–3346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita A, Buchholz DR, Shi YB 2003 Fusion protein of retinoic acid receptor α with promyelocytic leukemia protein or promyelocytic leukemia zinc-finger protein recruits N-CoR-TBLR1 corepressor complex to repress transcription in vivo. J Biol Chem 278:30788–30795 [DOI] [PubMed] [Google Scholar]