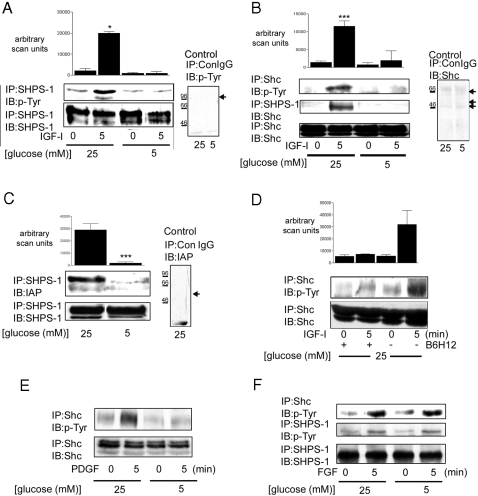
Figure 1.
Glucose Regulation of Shc Recruitment to SHPS-1, SHPS-1 Phosphorylation, and SHPS-1 Association with IAP
Cells were grown to confluency in either 25 or 5 mm glucose before overnight incubation in SFM with the appropriate glucose concentration. IGF-I (100 ng/ml) was added for the lengths of times indicated. A, SHPS-1 phosphorylation was determined by immunoprecipitating (IP) cell lysates with an anti-SHPS-1 antibody and then immunoblotting (IB) with an anti-phosphotyrosine antibody (p-Tyr) (top panel). The membrane was then stripped and reprobed with an anti-SHPS-1 antibody (bottom panel). To control for nonspecific precipitation of SHPS-1, cell lysates from SMC grown in 5 and 25 mm glucose was immunoprecipitated with nonimmune rabbit serum (ConIgG) and immunoblotted with the anti-phosphotyrosine antibody; a representative blot is shown in the panel labeled control, and the expected position of SHPS-1 is indicated with the black arrow. The positions of molecular weight standards are also shown. B, The extent of Shc phosphorylation was determined by immunoprecipitating one aliquot of the resulting cell lysates with an anti-Shc antibody and then immunoblotting with an anti-phosphotyrosine antibody (p-Tyr) (top panel). Shc association with SHPS-1 was determined by immunoprecipitating a second aliquot of the same lysate with an anti-SHPS-1 antibody and immunoprecipitating with an anti-Shc antibody (middle panel). Equal amounts of lysate were also immunoprecipitated and immunoblotted for total Shc protein (bottom panel). To control for nonspecific precipitation, cell lysate from SMC grown in both 5 and 25 mm glucose were immunoprecipitated with nonimmune rabbit serum (ConIgG) and then immunoblotted with the anti Shc antibody (the arrows indicate the small amount of p42/p52/p66 Shc that is precipitated nonspecifically); a representative blot is shown in the panel labeled control. The positions of molecular weight standards are also shown. C, SHPS-1 association with IAP was determined by immunoprecipitating cell lysates with an anti-SHPS-1 antibody and then immunoblotting with an anti-IAP monoclonal antibody, B6H12 (top panel). Membranes were stripped and reprobed with an anti-SHPS-1 antibody (bottom panel). To control for nonspecific precipitation of IAP, cell lysates from SMC grown in 25 mm glucose were subjected to immunoprecipitation with nonimmune rabbit serum and immunoblotted with B6H12; a representative blot is shown in the panel labeled control, and the expected position of IAP is shown with the black arrow. The positions of the molecular weight standards are also shown. D, Before treatment with IGF-I, SMC grown in 25 mm glucose were treated with the anti-IAP monoclonal antibody B6H12 (5 μg/ml) for 4 h. Shc phosphorylation was determined as described for B. The graphs show the mean result from three independent experiments displayed as arbitrary scanning units (A, B, and D: ***, P < 0.005, and *, P < 0.05 when the increase in response to IGF-I in cells maintained in 25 mm glucose is compared with the response of cells maintained in 5 mm glucose; C: ***, P < 0.005 when the association between IAP and SHPS-1 in 25 mm glucose is compared with that in 5 mm glucose). E, The extent of Shc phosphorylation was determined as for A except cells were treated with PDGF (10 ng/ml) for 5 min before lysis. F, SHPS-1 and Shc phosphorylation was determined as described for B except cells were treated with FGF (25 ng/ml) for 5 min before lysis.