Figure 3.
MMP-2 Cleaves IAP in 5 mm Glucose
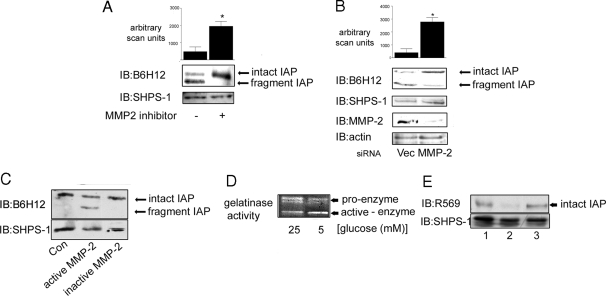
A, Cells were grown to confluency in medium containing 5 mm glucose before the initiation of the experiment then incubated overnight in SFM. The MMP-2/9 inhibitor (3 μg/ml) was added to some cultures for 4 h before terminating the experiment. After lysis and separation by SDS-PAGE, intact and fragmented IAP were visualized with the monoclonal anti-IAP antibody, B6H12 (top panel). Membranes were stripped and reprobed with an anti-SHPS-1 antibody. The graph shows the difference in intact IAP detected with B6H12 in SMC grown in 5 mm in the presence or absence of the MMP-2 inhibitor expressed as arbitrary scanning units (mean ± sd, n = 3; *, P < 0.05). B (upper panel), Cells transfected with the siRNA construct targeting MMP-2 (MMP-2) and the empty vector control (Vec) were grown to confluency in medium containing 5 mm glucose and then incubated overnight in SFM (5 mm) before lysis. IAP was visualized by immunoblotting (IB) with the anti-IAP monoclonal antibody B6H12. The graph shows the difference in intact IAP detected with B6H12 in siRNA MMP-2 SMC compared with empty vector control cells that had been grown in 5 mm glucose expressed as arbitrary scanning units (mean ± sd, n = 3; *, P < 0.05). B (lower panel), SMC that were transfected with either the siRNA-MMP-2 (MMP-2) or the empty vector control construct were screened by immunoblotting for MMP-2 protein levels. To control for loading, membranes were stripped and reprobed for β-actin. C, Membrane extracts prepared from SMC grown in 25 mm glucose were incubated with (+) or without (−) activated MMP-2 for 24 h. Membrane extracts were then separated by SDS-PAGE and IAP visualized by Western immunoblotting with the anti-IAP antibody B6H12. To control for the level of total protein, the blot was stripped and reprobed with an anti SHPS-1 antibody. The results shown are representative of three similar experiments with similar results. D, Conditioned media from SMC grown in 25 and 5 mm glucose were collected, concentrated, and then applied to a gelatin zymography gel. After staining with Coomassie blue, cleared areas were visualized as an indicator of MMP-2 gelatinase activity. The results shown are representative of three independent experiments with similar results. E (top panel), Membrane samples from SMC grown in 25 mm glucose were incubated with conditioned medium from SMC grown in 25 mm (lane 1) or 5 mm (lane 2) glucose or 5 mm glucose SFM from a cell-free culture (lane 3). After 24 h incubation at 37 C, the membrane extracts were separated by SDS-PAGE and immunoblotted with the anti-IAP antibody R569, specific for intact IAP. Reprobing with an anti SHPS-1 antibody demonstrated that there was no difference in the total amount of protein in each lane (bottom panel). The results shown are representative of three independent experiments with similar results.