Abstract
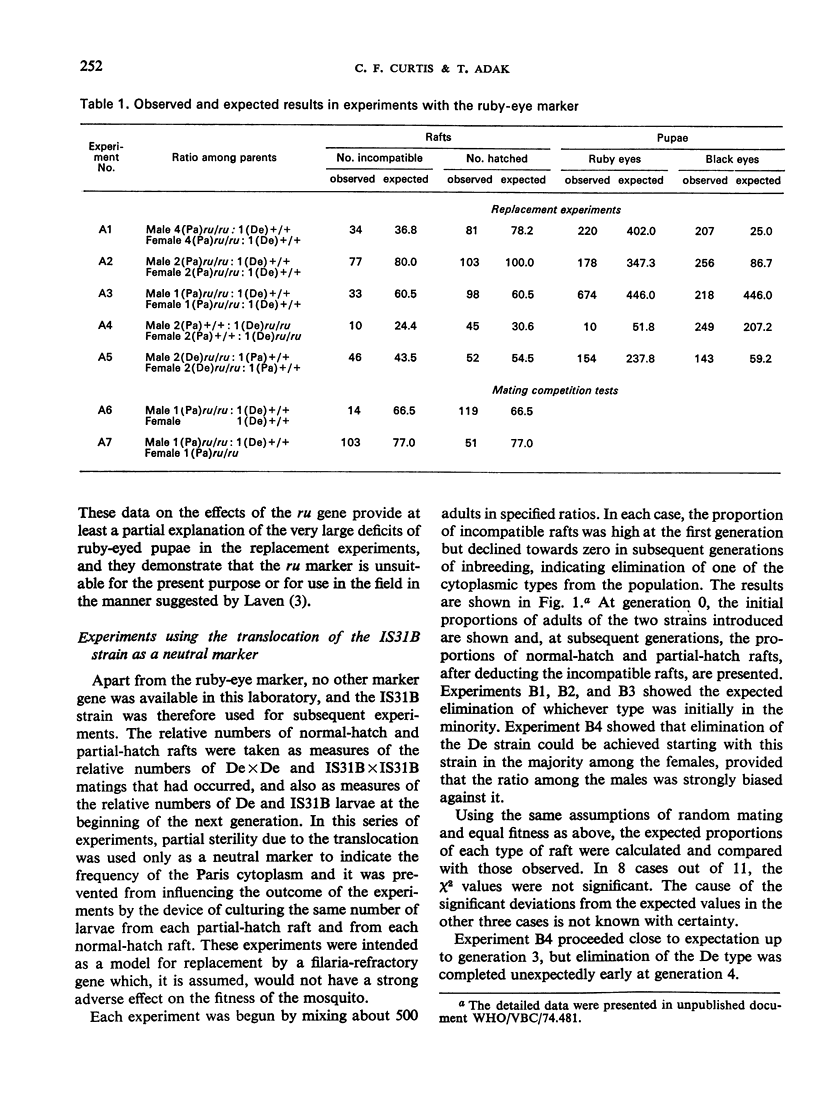
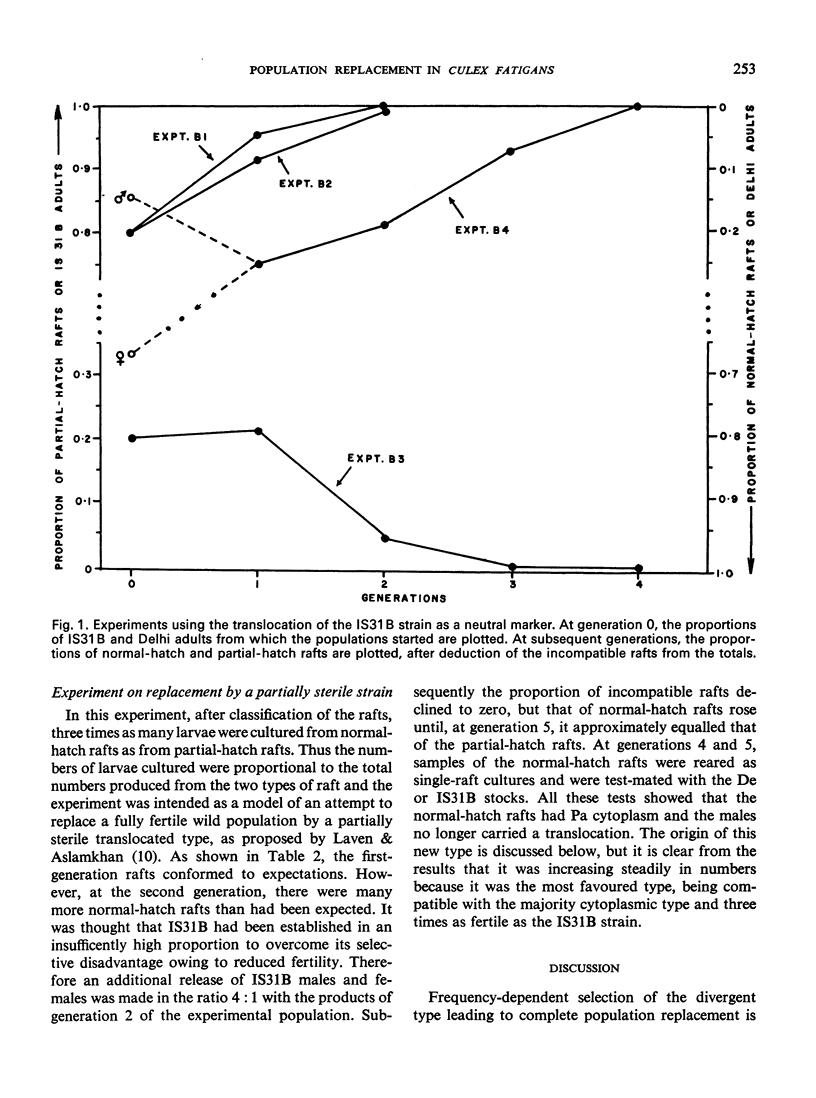
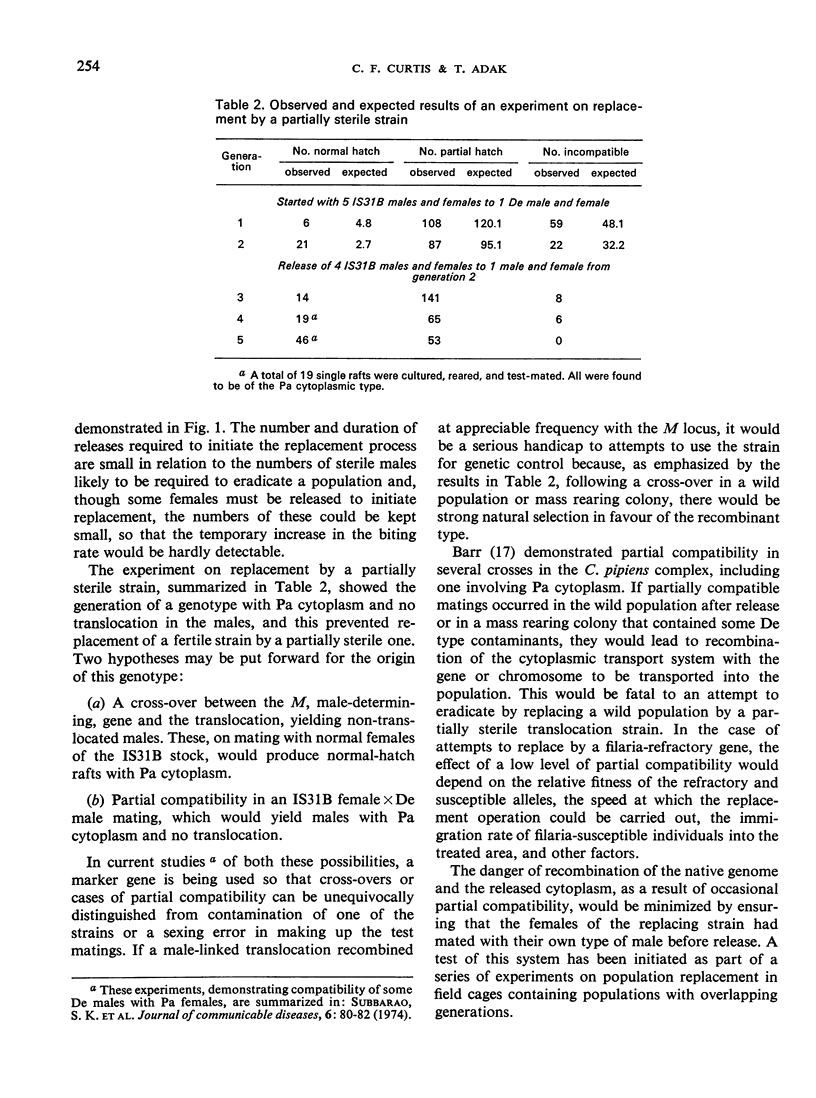
Bidirectional cytoplasmic incompatibility in the Culex pipiens complex appears to provide a mechanism for the replacement of a wild population by a strain refractory to filaria or a strain made partly sterile by a translocation. As a preliminary test of the feasibility of the replacement process, various ratios of strains with the cytoplasm of either Delhi or Paris, which are bidirectionally incompatible, were tested in laboratory cages. Where one strain was marked with the ruby-eye gene, this strain always declined in frequency in the next generation. In experiments in which the Paris strain was marked with a male-linked translocation complex, after 2-4 generations of breeding there was complete elimination of either the Paris or the Delhi type depending, as expected, on the relative frequencies of the two types with which the population began. In one experiment a type with Paris cytoplasm devoid of the translocation was found. This type increased in frequency in succeeding generations. The possible causes of origin of this type and its relevance to the practical use of the replacement principle are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Childress D. Changing population structure through the use of compound chromosomes. Genetics. 1972 Sep;72(1):183–186. doi: 10.1093/genetics/72.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis C. F., Hill W. G. Theoretical studies on the use of translocations for the control of Tsetse flies and other disease vectors. Theor Popul Biol. 1971 Mar;2(1):71–90. doi: 10.1016/0040-5809(71)90006-2. [DOI] [PubMed] [Google Scholar]
- Curtis C. F. Possible use of translocations to fix desirable genes in insect pest populations. Nature. 1968 Apr 27;218(5139):368–369. doi: 10.1038/218368a0. [DOI] [PubMed] [Google Scholar]
- Fitz-Earle M., Holm D. G., Suzuki D. T. Genetic control of insect population. I. Cage studies of chromosome replacement by compound autosomes in Drosophila melanogaster. Genetics. 1973 Jul;74(3):461–475. doi: 10.1093/genetics/74.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster G. G., Whitten M. J., Prout T., Gill R. Chromosome rearrangements for the control of insect pests. Science. 1972 May 26;176(4037):875–880. doi: 10.1126/science.176.4037.875. [DOI] [PubMed] [Google Scholar]
- Iltis W. G., Barr A. R., McClelland G. A., Myers C. M. The inheritance of yellow-larva and ruby-eye in Culex pipiens. Bull World Health Organ. 1965;33(1):123–128. [PMC free article] [PubMed] [Google Scholar]
- Laven H. Eradication of Culex pipiens fatigans through cytoplasmic incompatibility. Nature. 1967 Oct 28;216(5113):383–384. doi: 10.1038/216383a0. [DOI] [PubMed] [Google Scholar]
- Suzuki D. T. Temperature-sensitive mutations in Drosophila melanogaster. Science. 1970 Nov 13;170(3959):695–706. doi: 10.1126/science.170.3959.695. [DOI] [PubMed] [Google Scholar]
- Whitten M. J. Insect control by genetic manipulation of natural populations. Science. 1971 Feb 19;171(3972):682–684. doi: 10.1126/science.171.3972.682. [DOI] [PubMed] [Google Scholar]


