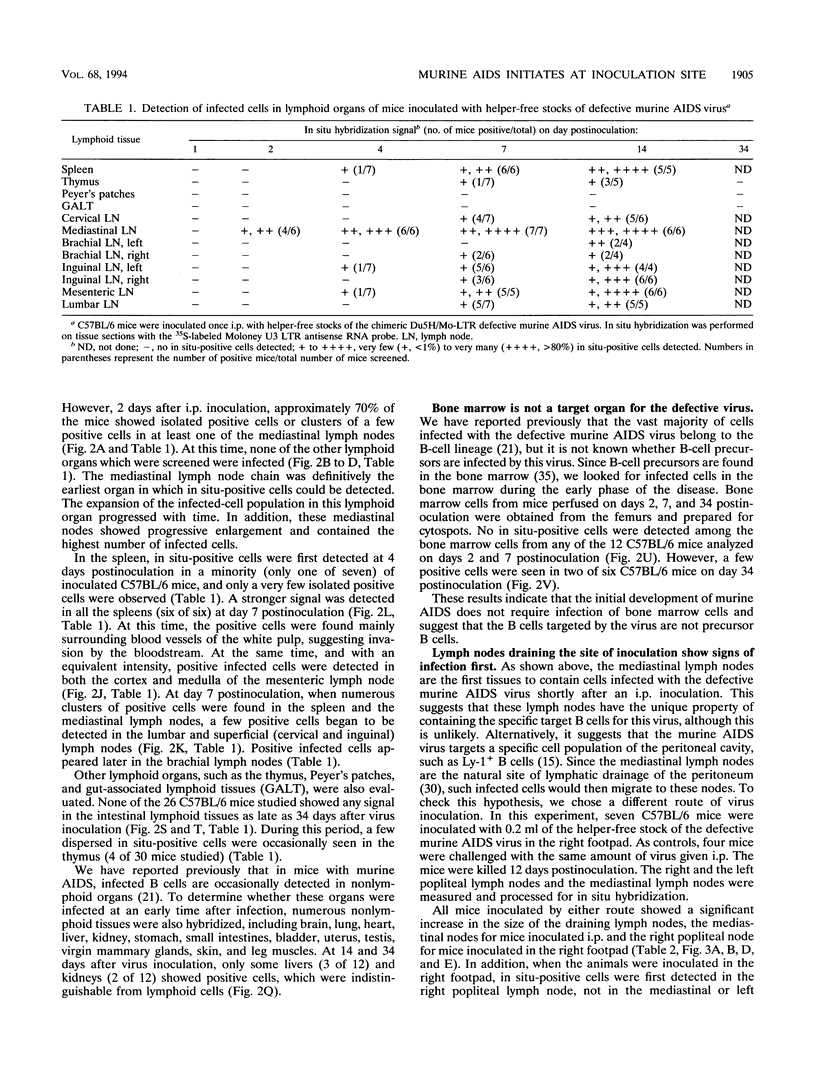
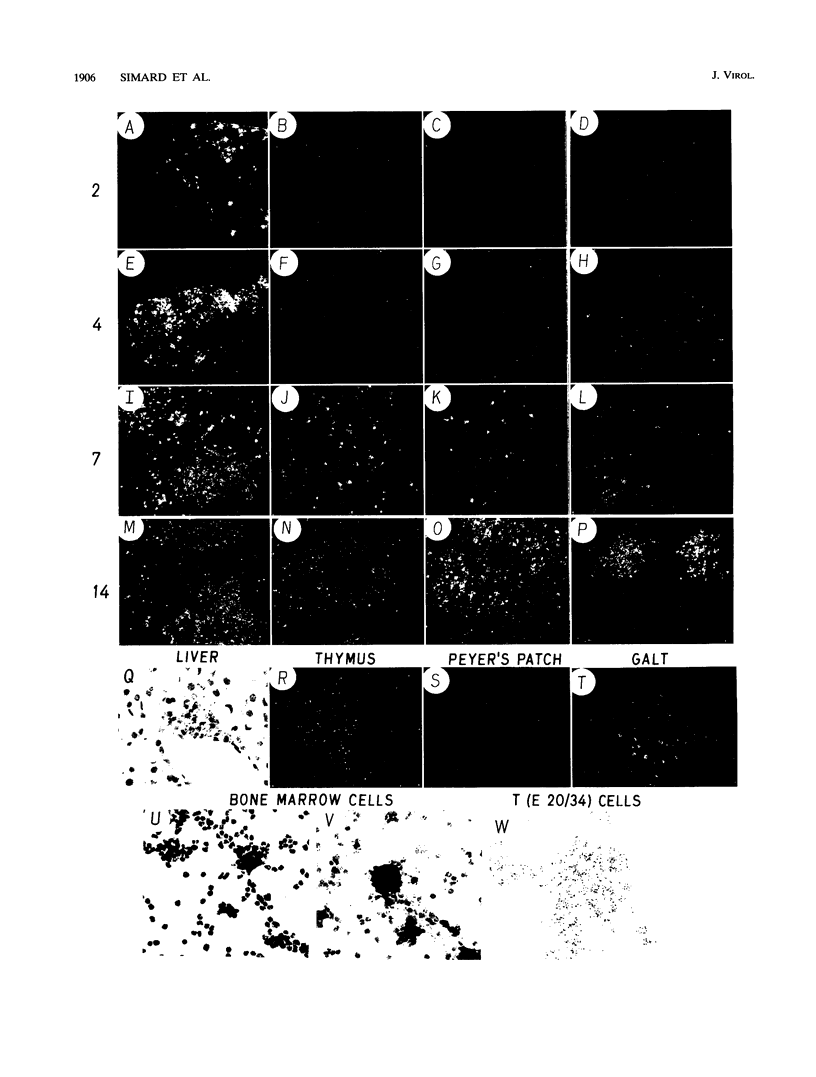
Abstract
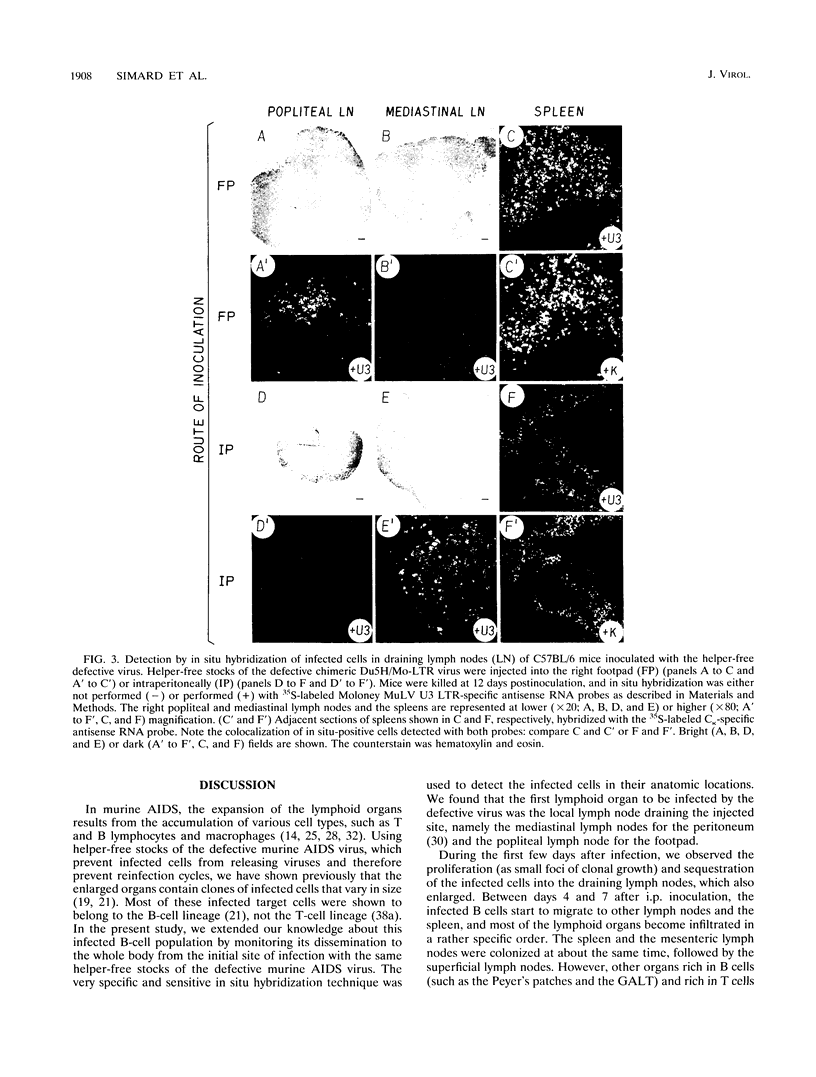
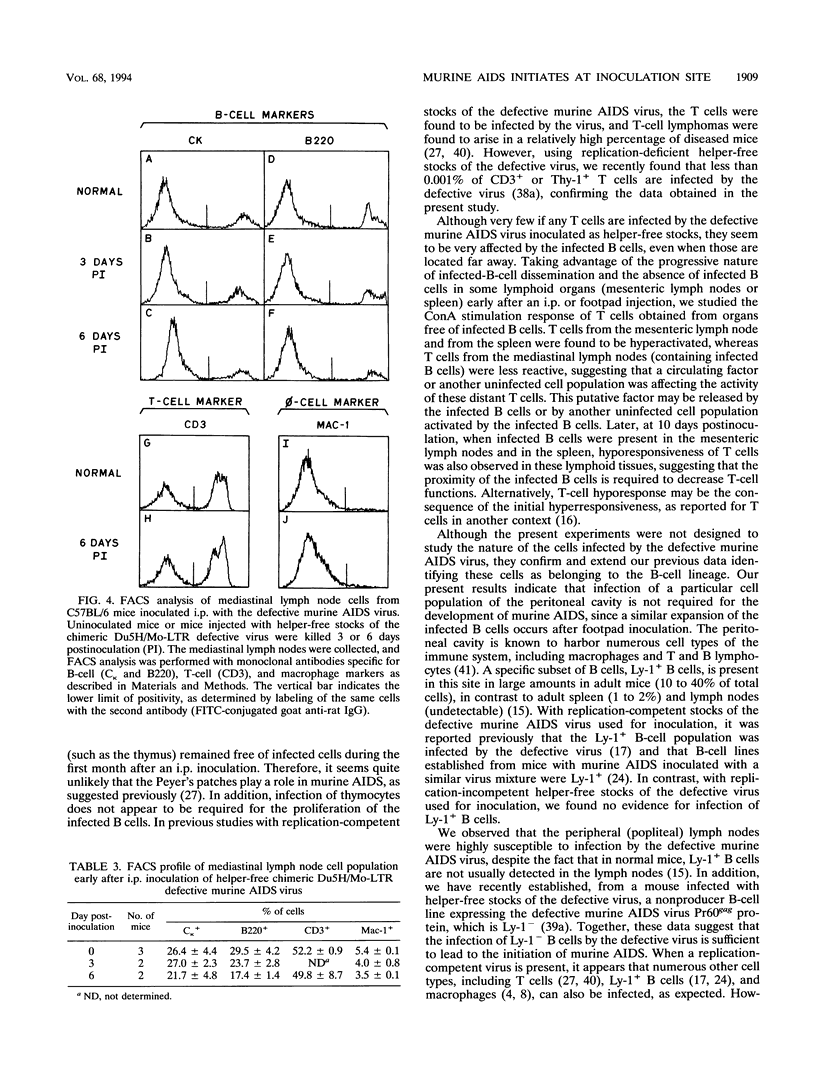
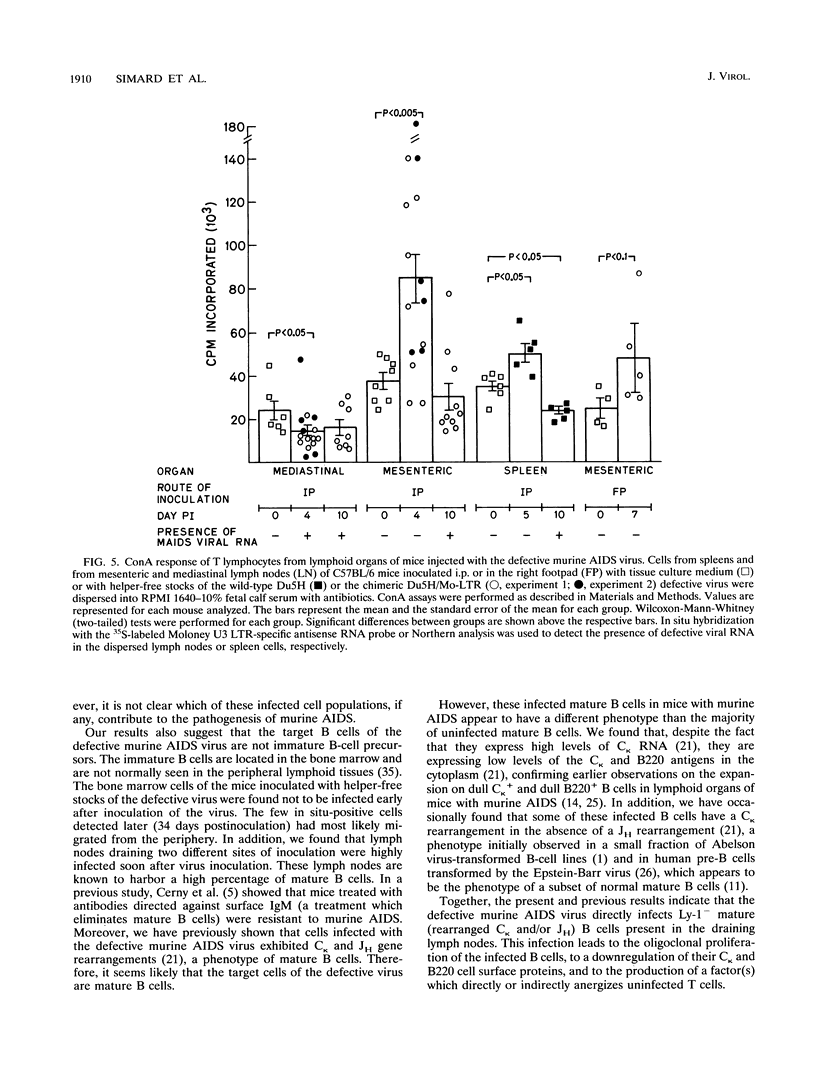
The infection of cells which belong to the B-cell lineage is thought to be the primary event leading to the phenotypic and functional alterations seen in the murine AIDS (M. Huang, C. Simard, D. Kay, and P. Jolicoeur, J. Virol. 65:6562-6571, 1991). Using in situ hybridization, we studied the time course of the anatomic distribution of the murine AIDS-infected B cells in C57BL/6 mice inoculated intraperitoneally or in the foot pad with helper-free stocks of the defective murine AIDS virus. The local lymph nodes draining the injection site (the mediastinal or popliteal lymph nodes) were the primary organs in which infected B cells could be detected. From this initial site, the proliferating infected B cells were found to migrate progressively to most of the other lymph nodes and to the spleen. The bone marrow cells (containing the precursor B cells) were not found to be infected by the virus. These results suggest that the defective murine AIDS virus infects mature Ly-1- B cells present in lymph nodes. We compared the concanavalin A response of the T cells at an early time postinoculation, before all lymphoid organs are infiltrated with infected B cells. In lymphoid organs free of infected B cells, T cells were found to be hyperresponsive. In lymphoid organs in which infected B cells were present, T cells were hyporesponsive. These data suggest that infected B cells influence distant T cells, maybe by the release of a circulating factor or through another uninfected cell population activated by the infected B cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F., Rosenberg N., Lewis S., Thomas E., Baltimore D. Organization and reorganization of immunoglobulin genes in A-MULV-transformed cells: rearrangement of heavy but not light chain genes. Cell. 1981 Dec;27(2 Pt 1):381–390. doi: 10.1016/0092-8674(81)90421-9. [DOI] [PubMed] [Google Scholar]
- Astier T., Guillemain B., Laigret F., Mamoun R., Duplan J. F. Serological Characterization of C-type retroviruses endogenous to the C57BL/6 mouse and isolated in tumours induced by radiation leukaemia virus (RadLV-Rs). J Gen Virol. 1982 Jul;61(Pt 50):55–63. doi: 10.1099/0022-1317-61-1-55. [DOI] [PubMed] [Google Scholar]
- Aziz D. C., Hanna Z., Jolicoeur P. Severe immunodeficiency disease induced by a defective murine leukaemia virus. Nature. 1989 Apr 6;338(6215):505–508. doi: 10.1038/338505a0. [DOI] [PubMed] [Google Scholar]
- Bilello J. A., Kort J. J., MacAuley C., Fredrickson T. N., Yetter R. A., Eiseman J. L. ZDV delays but does not prevent the transmission of MAIDS by LP-BM5 MuLV-infected macrophage-monocytes. J Acquir Immune Defic Syndr. 1992;5(6):571–576. [PubMed] [Google Scholar]
- Cerny A., Hügin A. W., Hardy R. R., Hayakawa K., Zinkernagel R. M., Makino M., Morse H. C., 3rd B cells are required for induction of T cell abnormalities in a murine retrovirus-induced immunodeficiency syndrome. J Exp Med. 1990 Jan 1;171(1):315–320. doi: 10.1084/jem.171.1.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Morse H. C., 3rd, Makino M., Ruscetti S. K., Hartley J. W. Defective virus is associated with induction of murine retrovirus-induced immunodeficiency syndrome. Proc Natl Acad Sci U S A. 1989 May;86(10):3862–3866. doi: 10.1073/pnas.86.10.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Sengupta D. N., Fredrickson T. N., Morse H. C., 3rd, Hartley J. W. Characteristics and contributions of defective, ecotropic, and mink cell focus-inducing viruses involved in a retrovirus-induced immunodeficiency syndrome of mice. J Virol. 1991 Aug;65(8):4232–4241. doi: 10.1128/jvi.65.8.4232-4241.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung S. C., Chattopadhyay S. K., Hartley J. W., Morse H. C., 3rd, Pitha P. M. Aberrant expression of cytokine genes in peritoneal macrophages from mice infected with LP-BM5 MuLV, a murine model of AIDS. J Immunol. 1991 Jan 1;146(1):121–127. [PubMed] [Google Scholar]
- Cheung S. C., Chattopadhyay S. K., Morse H. C., 3rd, Pitha P. M. Expression of defective virus and cytokine genes in murine AIDS. J Virol. 1991 Feb;65(2):823–828. doi: 10.1128/jvi.65.2.823-828.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Ehlich A., Schaal S., Gu H., Kitamura D., Müller W., Rajewsky K. Immunoglobulin heavy and light chain genes rearrange independently at early stages of B cell development. Cell. 1993 Mar 12;72(5):695–704. doi: 10.1016/0092-8674(93)90398-a. [DOI] [PubMed] [Google Scholar]
- Guillemain B., Astier T., Mamoun R., Duplan J. F. In vitro production and titration assays of B-tropic retroviruses isolated from C57BL mouse tumors induced by radiation leukemia virus (RadLV-Rs): effect of dexamethasone. Intervirology. 1980;13(2):65–73. doi: 10.1159/000149109. [DOI] [PubMed] [Google Scholar]
- Haas M., Reshef T. Non-thymic malignant lymphomas induced in C57BL/6 mice by cloned dualtropic viruses isolated from hematopoietic stromal cell lines. Eur J Cancer. 1980 Jul;16(7):909–917. doi: 10.1016/0014-2964(80)90329-1. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Fredrickson T. N., Yetter R. A., Makino M., Morse H. C., 3rd Retrovirus-induced murine acquired immunodeficiency syndrome: natural history of infection and differing susceptibility of inbred mouse strains. J Virol. 1989 Mar;63(3):1223–1231. doi: 10.1128/jvi.63.3.1223-1231.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R. Normal, autoimmune, and malignant CD5+ B cells: the Ly-1 B lineage? Annu Rev Immunol. 1988;6:197–218. doi: 10.1146/annurev.iy.06.040188.001213. [DOI] [PubMed] [Google Scholar]
- Herman A., Kappler J. W., Marrack P., Pullen A. M. Superantigens: mechanism of T-cell stimulation and role in immune responses. Annu Rev Immunol. 1991;9:745–772. doi: 10.1146/annurev.iy.09.040191.003525. [DOI] [PubMed] [Google Scholar]
- Hitoshi Y., Okada Y., Sonoda E., Tominaga A., Makino M., Suzuki K., Kinoshita J., Komuro K., Mizuochi T., Takatsu K. Delayed progression of a murine retrovirus-induced acquired immunodeficiency syndrome in X-linked immunodeficient mice. J Exp Med. 1993 Mar 1;177(3):621–626. doi: 10.1084/jem.177.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M., Jolicoeur P. Characterization of the gag/fusion protein encoded by the defective Duplan retrovirus inducing murine acquired immunodeficiency syndrome. J Virol. 1990 Dec;64(12):5764–5772. doi: 10.1128/jvi.64.12.5764-5772.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M., Simard C., Jolicoeur P. Immunodeficiency and clonal growth of target cells induced by helper-free defective retrovirus. Science. 1989 Dec 22;246(4937):1614–1617. doi: 10.1126/science.2480643. [DOI] [PubMed] [Google Scholar]
- Huang M., Simard C., Kay D. G., Jolicoeur P. The majority of cells infected with the defective murine AIDS virus belong to the B-cell lineage. J Virol. 1991 Dec;65(12):6562–6571. doi: 10.1128/jvi.65.12.6562-6571.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur P., Laperrière A., Beaulieu N. Efficient production of human immunodeficiency virus proteins in transgenic mice. J Virol. 1992 Jun;66(6):3904–3908. doi: 10.1128/jvi.66.6.3904-3908.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur P. Murine acquired immunodeficiency syndrome (MAIDS): an animal model to study the AIDS pathogenesis. FASEB J. 1991 Jul;5(10):2398–2405. doi: 10.1096/fasebj.5.10.2065888. [DOI] [PubMed] [Google Scholar]
- Kay D. G., Gravel C., Robitaille Y., Jolicoeur P. Retrovirus-induced spongiform myeloencephalopathy in mice: regional distribution of infected target cells and neuronal loss occurring in the absence of viral expression in neurons. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1281–1285. doi: 10.1073/pnas.88.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinken S. P., Fredrickson T. N., Hartley J. W., Yetter R. A., Morse H. C., 3rd Evolution of B cell lineage lymphomas in mice with a retrovirus-induced immunodeficiency syndrome, MAIDS. J Immunol. 1988 Feb 15;140(4):1123–1131. [PubMed] [Google Scholar]
- Klinman D. M., Morse H. C., 3rd Characteristics of B cell proliferation and activation in murine AIDS. J Immunol. 1989 Feb 15;142(4):1144–1149. [PubMed] [Google Scholar]
- Kubagawa H., Cooper M. D., Carroll A. J., Burrows P. D. Light-chain gene expression before heavy-chain gene rearrangement in pre-B cells transformed by Epstein-Barr virus. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2356–2360. doi: 10.1073/pnas.86.7.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo Y., Nakagawa Y., Kakimi K., Matsui H., Iwashiro M., Kuribayashi K., Masuda T., Hiai H., Hirama T., Yanagawa S. Presence of transplantable T-lymphoid cells in C57BL/6 mice infected with murine AIDS virus. J Virol. 1992 Sep;66(9):5691–5695. doi: 10.1128/jvi.66.9.5691-5695.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrand E., Daculsi R., Duplan J. F. Characteristics of the cell populations involved in extra-thymic lymphosarcoma induced in C57BL/6 mice by RadLV-Rs. Leuk Res. 1981;5(3):223–233. doi: 10.1016/0145-2126(81)90107-7. [DOI] [PubMed] [Google Scholar]
- Legrand E., Guillemain B., Daculsi R., Laigret F. Leukemogenic activity of B-ecotropic C-type retroviruses isolated from tumors induced by radiation leukemia virus (RadLV-RS) in C57BL/6 mice. Int J Cancer. 1982 Aug 15;30(2):241–247. doi: 10.1002/ijc.2910300219. [DOI] [PubMed] [Google Scholar]
- Marco A. J., Domingo M., Ruberte J., Carretero A., Briones V., Dominguez L. Lymphatic drainage of Listeria monocytogenes and Indian ink inoculated in the peritoneal cavity of the mouse. Lab Anim. 1992 Jul;26(3):200–205. doi: 10.1258/002367792780740549. [DOI] [PubMed] [Google Scholar]
- Morse H. C., 3rd, Chattopadhyay S. K., Makino M., Fredrickson T. N., Hügin A. W., Hartley J. W. Retrovirus-induced immunodeficiency in the mouse: MAIDS as a model for AIDS. AIDS. 1992 Jul;6(7):607–621. doi: 10.1097/00002030-199207000-00001. [DOI] [PubMed] [Google Scholar]
- Morse H. C., 3rd, Yetter R. A., Via C. S., Hardy R. R., Cerny A., Hayakawa K., Hugin A. W., Miller M. W., Holmes K. L., Shearer G. M. Functional and phenotypic alterations in T cell subsets during the course of MAIDS, a murine retrovirus-induced immunodeficiency syndrome. J Immunol. 1989 Aug 1;143(3):844–850. [PubMed] [Google Scholar]
- Mosier D. E. Animal models for retrovirus-induced immunodeficiency disease. Immunol Invest. 1986 May;15(3):233–261. doi: 10.3109/08820138609026687. [DOI] [PubMed] [Google Scholar]
- Mosier D. E., Yetter R. A., Morse H. C., 3rd Retroviral induction of acute lymphoproliferative disease and profound immunosuppression in adult C57BL/6 mice. J Exp Med. 1985 Apr 1;161(4):766–784. doi: 10.1084/jem.161.4.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmond D. G. Population dynamics of bone marrow B lymphocytes. Immunol Rev. 1986 Oct;93:103–124. doi: 10.1111/j.1600-065x.1986.tb01504.x. [DOI] [PubMed] [Google Scholar]
- Paquette Y., Doyon L., Laperrière A., Hanna Z., Ball J., Sekaly R. P., Jolicoeur P. A viral long terminal repeat expressed in CD4+CD8+ precursors is downregulated in mature peripheral CD4-CD8+ or CD4+CD8- T cells. Mol Cell Biol. 1992 Aug;12(8):3522–3530. doi: 10.1128/mcb.12.8.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette Y., Kay D. G., Rassart E., Robitaille Y., Jolicoeur P. Substitution of the U3 long terminal repeat region of the neurotropic Cas-Br-E retrovirus affects its disease-inducing potential. J Virol. 1990 Aug;64(8):3742–3752. doi: 10.1128/jvi.64.8.3742-3752.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M., Lerner R. A., Sutcliffe J. G. Nucleotide sequence of Moloney murine leukaemia virus. Nature. 1981 Oct 15;293(5833):543–548. doi: 10.1038/293543a0. [DOI] [PubMed] [Google Scholar]
- Simard C., Jolicoeur P. The effect of anti-neoplastic drugs on murine acquired immunodeficiency syndrome. Science. 1991 Jan 18;251(4991):305–308. doi: 10.1126/science.1987646. [DOI] [PubMed] [Google Scholar]
- Tang Y., Fredrickson T. N., Chattopadhyay S. K., Hartley J. W., Morse H. C., 3rd Lymphomas in mice with retrovirus-induced immunodeficiency. Curr Top Microbiol Immunol. 1992;182:395–398. doi: 10.1007/978-3-642-77633-5_50. [DOI] [PubMed] [Google Scholar]
- Yaffe P., Yoffey J. M. Phagocytic lymphoid cells and transitional cells in the peritoneal cavity. J Anat. 1982 Jun;134(Pt 4):729–740. [PMC free article] [PubMed] [Google Scholar]
- Yetter R. A., Buller R. M., Lee J. S., Elkins K. L., Mosier D. E., Fredrickson T. N., Morse H. C., 3rd CD4+ T cells are required for development of a murine retrovirus-induced immunodeficiency syndrome (MAIDS). J Exp Med. 1988 Aug 1;168(2):623–635. doi: 10.1084/jem.168.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]