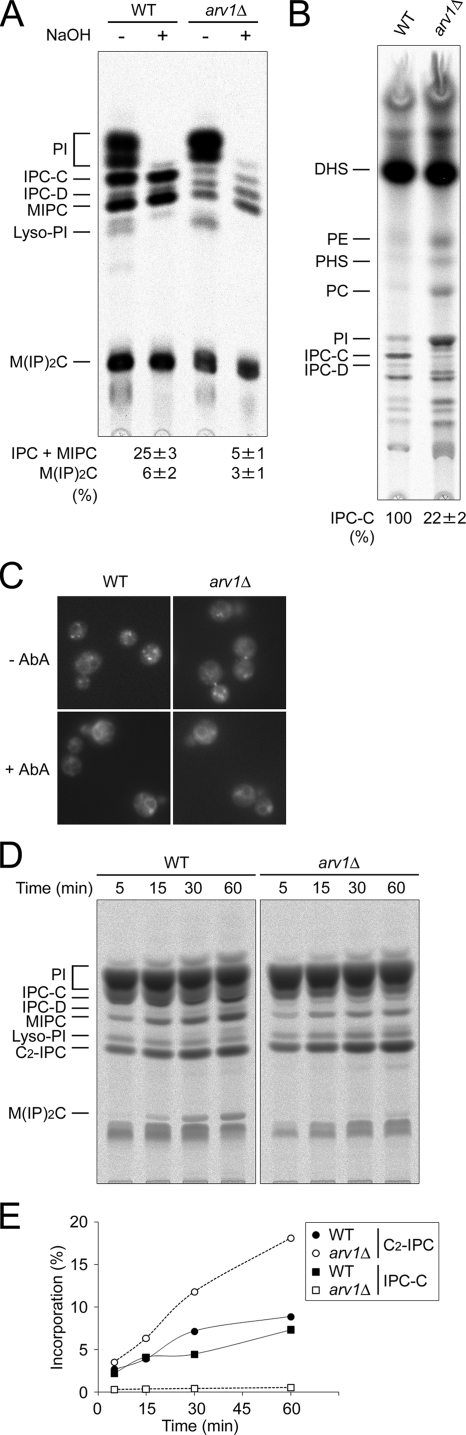
Figure 1.
The arv1Δ mutant is defective in ceramide transport from the ER to the Golgi. (A and B) Wild-type (RH6082) and arv1Δ (RH6078) strains were labeled with [3H]myo-inositol (A) or with [3H]DHS (B) for 2 h at 25°C. The [3H]myo-inositol–labeled lipids were subjected (+) or not (−) to mild alkaline hydrolysis with NaOH. (C) The same strains as in A were labeled with C6-NBD-ceramide for 15 min at 25°C, with or without AbA as described in Materials and Methods, and the staining was visualized by fluorescence microscopy. (D) The same strains were also labeled with [3H]myo-inositol for the indicated times at 25°C in the presence of C2-ceramide. The labeled lipid products were applied to TLC plates using solvent system I (A and D) or solvent system II (B). Lipids were identified by their mobility and resistance to mild-base treatment. The band, which was identified as C2-IPC, appeared only when the precursor C2-ceramide was added, and the appearance was resistant to mild-base treatment and sensitive to the addition of AbA (not shown). Incorporation (%) into sphingolipids was determined as the percentage of total radioactivity into all lipids containing inositol (A and D). Incorporation of [3H]DHS into IPC-C was quantified, and the relative amounts were calculated by setting the amounts in wild-type cells to 100% (B). Results of a typical experiment are shown. Data in A and B are means ± SE for six and four independent experiments, respectively, and in E are quantification of D. PI, phosphatidylinositol; IPC-C and -D, inositolphosphorylceramide subclasses C and D; MIPC, mannosyldi(inositolphosphoryl) ceramide; Lyso-PI, lyso-phosphatidylinositol; DHS, dihydrosphingosine; PE, phosphatidylethanolamine; PHS, phytosphingosine; PC, phosphatidylcholine.