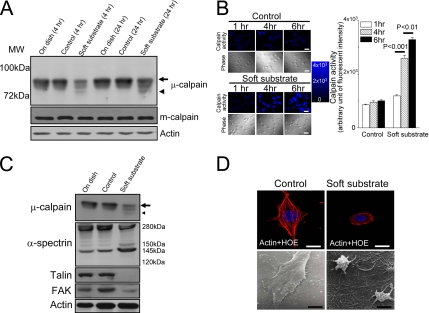
Figure 2.
Soft substrate induces μ-calpain activation and disrupts the cellular structure of normal cervical epithelial cells. (A) Normal cervical epithelial cells were cultured on various conditions. Cell lysates were harvested at the indicated time points. Arrow and arrowhead, the inactive and active catalytic μ-calpain subunit, respectively. (B) Left panel, representative pictures of time courses of CMAC fluorescent intensity in various culture conditions. Normal cervical epithelial cells were cultured on control condition or on soft substrate for 1, 4, and 6 h. Intracellular calpain activities were measured by using a fluorogenic membrane-permeable calpain substrate t-Boc-LM-CMAC. Cleavage of t-Boc-LM-CMAC by active calpain induced the retention of CMAC portion of the molecule in the cells and resulted in increased fluorescence. Scale bar, 30 μm. Right panel, quantification of the arbitrary unit of fluorescent intensity, which represents the calpain activity. Each column represents mean ± SEM from at least 150 different cells. (C) Soft substrate–induced cleavage of α-spectrin and degraded adhesion molecules. Normal cervical epithelial cells were cultured on various conditions for 6–8 h. Small arrow and small arrowhead, the inactive and active catalytic μ-calpain subunit, respectively. (D) Soft substrate–induced disorganization of actin filaments in normal cervical epithelial cells. Top panel, cells were cultured on control condition or on soft substrate for 8–10 h, and immunofluorescence study was performed to detect actin filaments and nucleus by immunoreaction with phalloidin-Alexa 594 and Hoechst 33258 (HOE), respectively. Scale bars, 10 μm. Bottom panel, morphology of normal cervical epithelial cells cultured on control condition or on soft substrate for 8–10 h, detected by scanning electron microscope. The SEM picture in the control culture condition shows 4–5 confluent cells, but the SEM picture in the soft substrate culture condition shows two round-up cells. Scale bars, 4 μm.