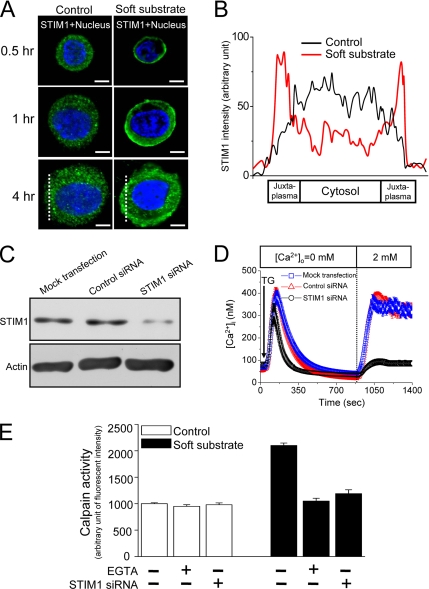
Figure 6.
STIM1-mediated Ca2+ influx activates calpain. (A) Soft substrate induces the translocation of STIM1. Normal cervical epithelial cells were cultured on different substrates for 0.5, 1, and 4 h, and the confocal images were then taken by immunofluorescent staining. A representative image of five different experiments. Scale bar, 8 μm. Dashed lines indicate the area of quantitative analyses of STIM1 fluorescent intensity shown in B. (B) Quantitative fluorescent analysis of cellular STIM1 distribution. Each trace is the mean of fluorescent intensity from five different experiments. (C and D) Knockdown of STIM1 protein inhibits store-operated Ca2+ influx. Normal cervical epithelial cells were transfected with vehicle alone (Mock), control nontargeting siRNA (Control siRNA, 100 nM), or STIM1 siRNA (100 nM) for 48 h. Then at 48 h after transfection, the cells were harvested for analysis of protein expression by immunoblotting (C), and the single-cell [Ca2+]i measurement was studied (D). TG, 2 μM thapsigargin. (E) Soft substrate–induced μ-calpain activation was inhibited by blockade of Ca2+ influx with 1.5 mM EGTA and knockdown of STIM1 by siRNA. Normal cervical epithelial cells were cultured on collagen gel–coated dish (control) or on collagen gel (soft substrate) for 4 h. Intracellular calpain activities were measured by using a fluorogenic membrane-permeable calpain substrate t-Boc-LM-CMAC. Each column represents mean ± SEM (n = 6). EGTA, ethyleneglycol-bis(2-aminoethylether)-N,N,N′,N′-tetra-acetic acid.