Fig. 2.
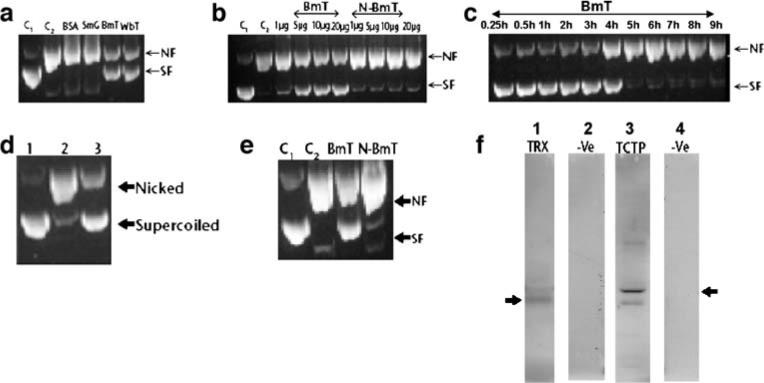
DNA nicking assay (a–e). Approximately 200 ng of variously treated DNA was resolved on a 1% agarose gel followed by staining with Etbr, and bands were visualized using a UV transilluminator. a ϕX174 DNA was incubated in the presence of rBmTCTP that was reduced by DTT (reduced BmT) or rWbTCTP that was reduced by DTT (reduced WbT). Results show that the reduced form of BmT and WbT substantially decreased the formation of nicked DNA (NF), leaving majority of the DNA in the supercoiled form (SF) similar to that of the untreated ϕX174 DNA (C1). However, incubation of ϕX174 DNA in MFO buffer alone (C2) or in the presence of BSA or a control recombinant protein, S. mansoni G box binding factor (SmG), resulted in significant nicking of the DNA. b Dose-dependent inhibition of DNA nicking by reduced BmT. Addition of N-ethylenemaleimide modified TCTP (N-BmT) instead of reduced BmT almost completely reversed its DNA protecting ability. Increasing the concentration of reduced BmT in the reaction mixture substantially decreased the nicked form of DNA. However, addition of N-BmT had no protective effect. c Time kinetics studies showed that the DNA protecting effect of reduced rBmTCTP is time-dependent and is present up to 4 h after initiating the incubation. d TCTP reduced by Trx system also efficiently protects DNA from oxidative damage compared to unreduced rBmTCTP Lane 1 Untreated ϕX174 DNA, Lane 2 ϕX174 DNA incubated with unreduced rBmTCTP, Lane 3 ϕX174 DNA incubated with rBmTCTP reduced with Trx. e Addition of DTT-reduced rBmTCTP conferred protection from oxidative damage, and this effect was reversed when N-BmT was used instead of reduced BmT. f Five micrograms of E. coli Trx was resolved on 15% SDS-PAGE, transferred to nitrocellulose membrane, probed with biotinylated rBmTCTP, and developed by ECL. Arrow shows that rBmTCTP interacts with Trx (lane 1). Similarly, 5 μg of rBmTCTP was resolved on 12% SDS-PAGE, transferred to nitrocellulose membrane, incubated with Trx, and probed with a horse radish peroxidase labeled-rabbit anti-Trx antibody and color-developed using an ECL kit (lane 3). Arrow shows the binding of Trx with rBmTCTP. Lanes 2 and 4 were negative controls using BSA. Data presented are representative of one of three similar experiments
