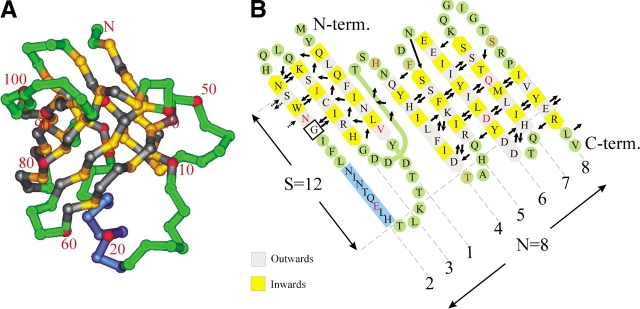
Figure 1.
(A) Cα trace of staphostatin B from the side. Residues in β-strands are yellow and gray, depending on whether they face the inside or outside of the barrel, respectively. Loop regions and turns are green, and the helix is dark blue. Every tenth residue is labeled and marked red. (B) Main chain hydrogen bonding arrangement in staphostatin B as assigned with the program O (Jones et al. 1991). Only hydrogen bonds to or from residues within β-strands are represented and are drawn as arrows pointing from donor to acceptor. Secondary structure assignment was done with dssp (Kabsch and Sander 1983), except for strand β6. Note that for consistency with A, the unwrapped barrel is seen from what was “inside,” resulting in the unconventional slant of β-strands to the left.