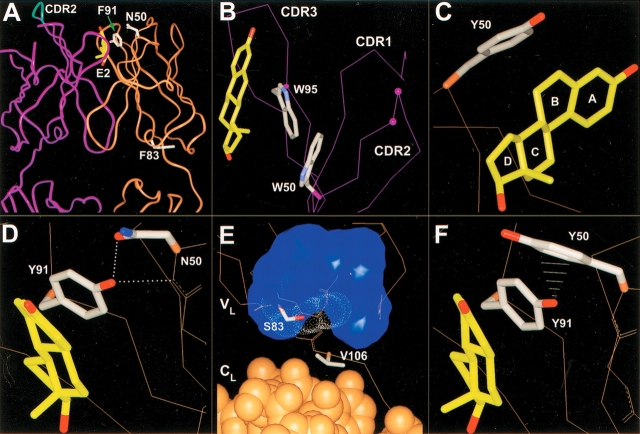
Figure 2.
Antibody 57-2 mutations. In all the figures, the heavy-chain is colored purple; the light-chain, brown. The side chains of the important residues are drawn. For the other parts of the protein, only the Cα-trace or the backbone heavy atoms are shown, if not mentioned. (A) The variable domain and a part of the constant domain of the antibody 57-2. The estradiol molecule and the side chains of light-chain wild-type residues targeted by point mutations are shown. The tip of the heavy-chain CDR2 targeted by a four-amino-acid insertion is shown as cyan. (B) The insertion site in CDR2 of heavy-chain. The insertion was introduced between the residues labeled with purple balls. Only the Cα-atoms are shown, except for the tryptophans W50H and W95H, for which side chains are also shown. (C) Mutation N50LY. The side chain of the mutated residue is shown and the lettering of the steroid rings is indicated. (D) Mutation F91LY. The dotted lines represent hydrogen bonds. (E) Mutation F83LS. The van der Waals surface of the side chain of Phe83L replaced by the mutation is indicated with white dots. The blue surface shows the volume occupied by the hydrophobic side chains of the residues situated in the vicinity of the residue 83L. The light-chain constant domain atoms are drawn as CPK-models, whereas for the variable domain only Cα-trace or the backbone heavy atoms is shown. (F) The double mutation N50LY and F91LY. The white lines represent the close contacts (∼2.7 to 3.1 Å) between the ηOH of Tyr91L and the aromatic carbons of Tyr50L.