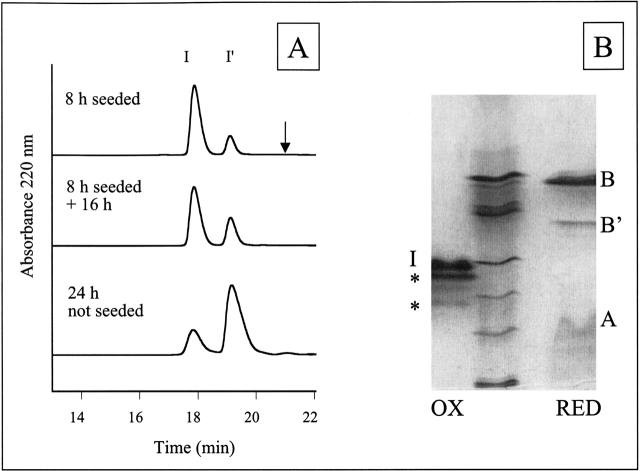
Figure 2.
HPLC and IEF of solubilized insulin fibrils. (A) HPLC traces of solubilized insulin fibrils under nonreducing HPLC conditions reveal two peaks, I and I′. The peak labeled I corresponds to unmodified insulin and insulin with B-chain deamidation (determined by IEF). The I′ peak corresponds to insulin with A-chain deamidation and insulin with both A-chain deamidation and B-chain deamidation (IEF). The arrow indicates the expected relative retention time of the A8–A9 split product (Brange et al. 1992). The extent of Asn A21 deamidation can be approximated by the relative ratio of I′ to I. The Asn A21 deamidation in the 24 h unseeded fibrils is greater than the modification of the 8 h seeded + 16 h fibrils, indicating less chemical modification in the fibrils than in solution. (B) IEF gel electrophoresis of solubilized insulin amyloid fibrils formed by incubation for 24 h. IEF was performed under nonreducing (lane 1, OX) and reducing (lane 3, RED) conditions and compared with IEF marker proteins (lane 2) corresponding to pI 7.2, 6.4, 5.9, 5.1, 4.6, and 3.3 from top to bottom. Bands that occur in the same position as for an untreated insulin sample are labeled with I (insulin), A (A-chain), or B (B-chain) respectively. IEF under nonreducing conditions reveals insulin (calc. pI 5.4) and two additional bands. The dark band closest to insulin is assigned to insulin deamidated at position Asn A21 and the fainter bands to deamidation at Asn B3 (based on previous assignment by Brange et al. 1992) or insulin deamidated at more than one position (a single deamidation would reduce the pI by 0.4–0.5 and double deamidation by 0.7–0.8). Under reducing conditions, the B-chain (calc. pI 6.9) and a deamidated form of the B-chain (a single deamidation would reduce the pI by 0.9) are clearly distinguishable. These two bands were sequenced to reveal the presence of deamidated Asn B3 and Gln B4. The A-chain (both in control insulin solutions and in fibrils) smears on the gel, possibly due to reoxidation.