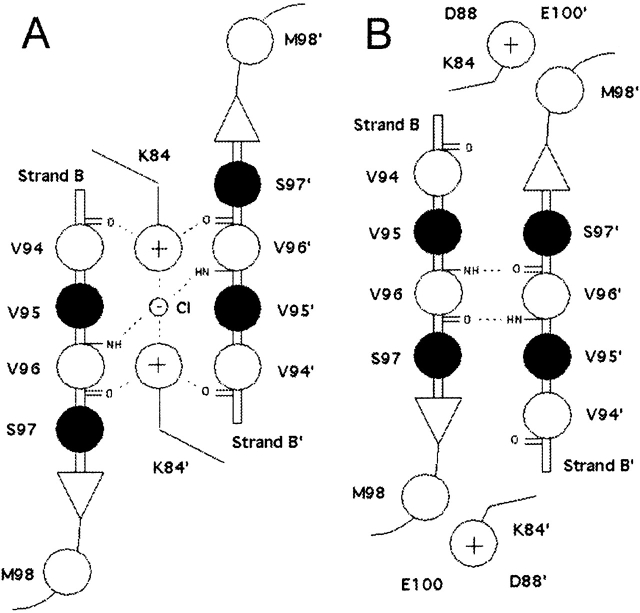
Figure 5.
Schematic representation of N-subdomain monomer–monomer interface in the repressed (A) and the induced (B) conformations. The view is from above the N-subdomains looking down into the interface. Filled circles indicate the side chain is above the plane of the β-sheets; open circles, the side chain lies below the plane. Note that residue 84 is in the plane of the β-sheets, even though its side chain is white. The repressed interface (A) is dominated by hydrogen bond and electrostatic interactions (dotted lines) that stabilize the K84 pair and chloride anion in the space located between the two β-strands. In the induced conformation (B), the K84 side chains are outside the interface, allowing the backbones of V96 and V96’ to form hydrogen bonds, creating an antiparallel β-sheet across the interface. V94 and V96 are also members of the N-subdomain hydrophobic group (see Fig. 1B ▶).