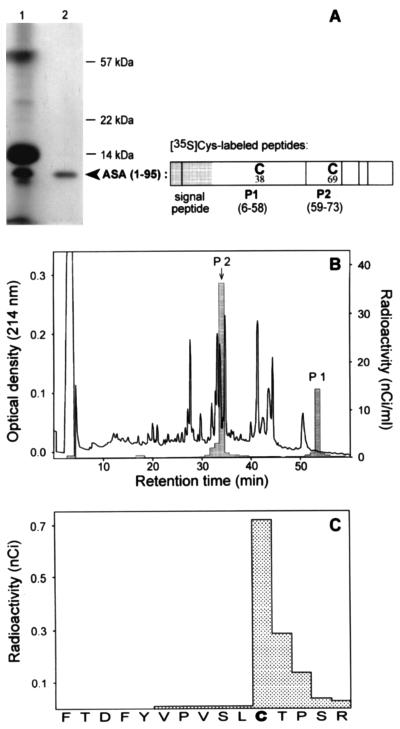
Figure 1.
Incorporation of cysteine into the primary ASA translation product at position 69. The N-terminal 95 residues of ASA were expressed in vitro in the presence of [35S]cysteine using a truncated run-off transcript as a message. The translation products were analyzed by SDS/PAGE and fluorography (A). From the crude translation products of the reticulocyte lysate (lane 1) the ASA (1–95) nascent polypeptide was purified by sedimentation of ribosomes (lane 2). The distribution of cysteines within ASA (1–95) and its tryptic peptides 1 and 2 is shown in the scheme. Following release from the ribosomes by addition of puromycin, radiolabeled ASA (1–95) (about 10 nCi per cysteine; 1 Ci = 37 GBq) was mixed with 30 μg of unlabeled ASA protein, serving as carrier, and subjected to reductive carboxymethylation, digestion with trypsin and separation of tryptic peptides by RP-HPLC (B). The cysteine containing form of peptide 2 (position indicated by an arrow) and the FGly containing form of peptide 2 were identified by mass spectrometry. The 35S-labeled peptides were localized by liquid scintillation counting of HPLC fractions (see histogram). [35S]P2 was identified by radiosequencing demonstrating that radioactivity was incorporated in position 69 (sequencing cycle 11) (C). The sequence of P2 is given on the abscissa. [35S]P2 of the translation product and the cysteine containing P2 of the carrier protein coeluted in the same fraction. 35S-labeled peptide 1 eluted at an 8% higher acetonitrile concentration as compared with peptide 1 of the carrier protein. This can be explained by the N-terminal extension of [35S]peptide 1 by the hydrophobic residues 6–18, which are part of the signal peptide that is absent in the carrier ASA.