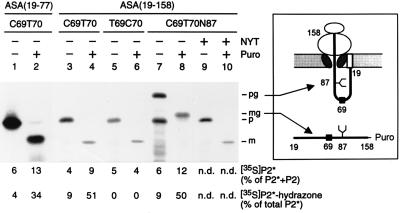
Figure 5.
In vitro modification of nascent ASA polypeptides occurs at a posttranslational or late cotranslational stage. In vitro translation reactions in the presence of [35S]methionine and rough microsomes (see Fig. 2) were programmed with run-off transcripts coding for a fusion of the preprolactin signal peptide and the N-terminal residues 19–77 (lanes 1–2) or 19–158 (lanes 3–10) of the following ASA constructs shown in Fig. 4: construct 2 (lanes 1–4), construct 3 (lanes 5–6) or construct 2 carrying the N-glycosylation signal N87Y88T89 (lanes 7–10). After translation the microsomes were isolated and one half was kept on ice, while the other half was incubated with puromycin (Puro), as indicated. One tenth of each sample was analyzed by SDS/PAGE and fluorography; note that lanes 1 and 2 were run in a different gel system (see Methods). The remaining part was stopped with guanidine hydrochloride and analyzed for modification of peptide 2, as explained in Fig. 4 (n.d., not determined). The polypeptides visible in the fluorograms represent the precursor (p), mature (m), glycosylated precursor (pg), or glycosylated mature (mg) form of the translation products. The identification of the pg (lane 7) and the mg form (lane 8) is confirmed by the observation that in the presence of 0.1 mM glycosylation inhibitor peptide NYT (16) the nonglycosylated p (lane 9) and m (lane 10) forms were synthesized. Note that the mature form contains one methionine and the precursor form contains two methionines. The scheme depicts the topology of the glycosylated precursor arrested during translocation and exposing Cys-69 inside the ER (Upper). The glycosylated mature form is released into the ER-lumen as a peptidyl-puromycin product (Lower). The numbers refer to the ASA residues. The symbols at positions 69 and 87 indicate the sites of modification and N-glycosylation, respectively. The signal peptide is shown as an open box.