Fig. 2.
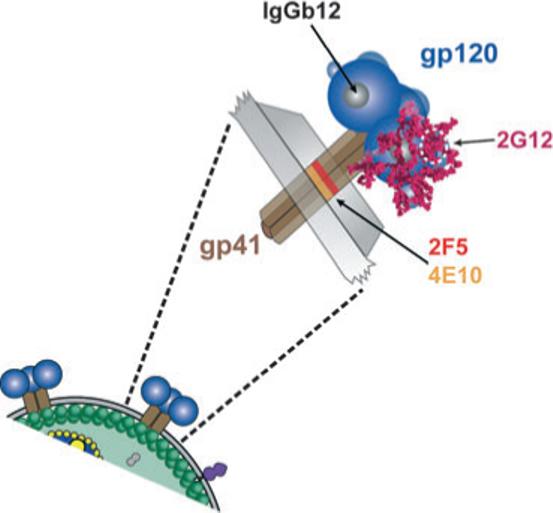
Schematic representation of the functional spike and the binding sites of broadly neutralizing antibodies on the viral envelope glycoproteins. The exterior envelope glyco-protein, gp120 (blue), and the transmembrane glycoprotein, gp41 (brown) are shown as noncovalently associated trimers on the surface of the virus (below) and expanded to reveal more details of the spike (above). The membrane proximal epitopes for the two broadly neutralizing gp41 antibodies; 4E10 (orange) and 2F5 (red) are shown on the trimeric stalk of gp41 external to the viral membrane. The two broadly neutralizing gp120 antibodies are 2G12 and IgGb12. 2G12 recognizes a carbohydrate cluster (encircled in white dashes) on the N-linked glycans (magenta) of gp120, which are shown on one gp120 monomer. The antibody IgGb12 recognizes a discontinuous epitope that overlaps with the recessed CD4-binding site on gp120 (grey area) and is shown on one monomer.
