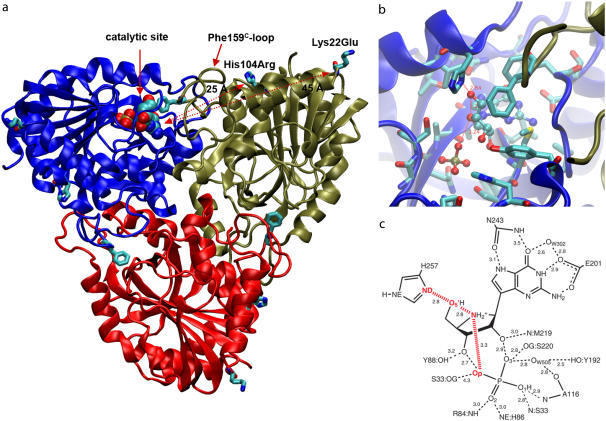
FIGURE 2.
(a) Asymmetric unit of trimer hPNP with the catalytic site of subunit-A located near the trimer interface and covered with the loop including Phe-159C of subunit C. Mutated residues His104Arg and Lys22Glu are located 25 Å and 45 Å away from the catalytic site of the adjacent subunit and 26 Å and 32 Å from the catalytic site of their own subunit. (b) The active site residues near ImmG and the phosphate nucleophile. (c) Structure of ImmG at the catalytic site of hPNP. Active site residues in contact with ImmG and the nucleophile phosphate are shown. The ImmG:O5′, ImmG:N4′, H257:Nδ, and 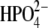 :Op atoms are highlighted in red.
:Op atoms are highlighted in red.